AbstractPurposePeritoneal recurrence is one of the most common patterns of recurrence after gastric cancer surgery and it has a poor prognosis despite all efforts. The aim of this study is to evaluate the prognostic impact of early postoperative intraperitoneal chemotherapy (EPIC) after surgery with curative intent for macroscopically serosa-invading gastric cancer patients.
Materials and MethodsThe records of 245 patients under the age of 70 were reviewed. These patients were suffering from macroscopically seroa-invading gastric cancer and they underwent curative surgery from 1995 to 2004 at the Kyungpook National University Hospital, Daegu, Korea. The overall survival, gastric cancer-specific survival, complications, and patterns of recurrence were compared between the patients who were treated with EPIC and those who were not.
ResultsEPIC was administered to 65 patients, and the remaining 180 patients did not receive this treatment. The 5-year overall and gastric cancer-specific survival rates for the EPIC group were 47.4% and 53.1%, respectively, and those for the non-EPIC group were 26.7% and 29.7%, respectively (p=0.012 for overall survival and p=0.011 for gastric cancer-specific survival). The rates of peritoneal recurrence for the EPIC group and the non-EPIC group were 18.5% and 32.2%, respectively (p=0.038). There were no significant differences in the morbidity or mortality between the two groups. Based on a multivariate analysis of the factors with prognostic significance in univariate analyses, EPIC, pathological lymph node metastasis, differentiation, and the extent of gastric resection were independent prognostic factors.
IntroductionAs more gastric cancer patients are diagnosed during the early stage of the disease, the survival of gastric cancer patients has improved recently. However, gastric cancer is still a major cause of cancer-related deaths worldwide. This is partly because a large portion of patients are diagnosed during the late stages and partly due to the fact that curative resection cannot guarantee an absolute cure for the disease. Recurrence is the most serious threat for patients who have undergone curative surgery. Systemic chemotherapy has been used in attempts to prevent recurrence; however, the results of these trials were not satisfactory [1,2]. Among the different modes of recurrence, peritoneal carcinomatosis is a common pattern of treatment failure, and serosal exposure is the most potent risk factor for peritoneal carcinomatosis [3]. Although we have not reached a unanimous consensus, favorable reports describing the outcome of intraperitoneal chemotherapy (IPC) have been published [4-8].
In this study, we review the clinicopathological characteristics of patients and the effects of early postoperative intraperitoneal chemotherapy (EPIC) on the overall and gastric cancer-specific survival, as well as on the patterns of recurrence for gastric cancer patients with macroscopic serosal invasion.
Materials and Methods1. PatientsA total of 495 gastrectomies were performed on patients with histologically proven gastric cancer with serial invasion, according to intraoperative macroscopic findings, in the period between 1995 and 2004 at the Kyungpook National University Hospital. The enrolled patients had good performance statuses, white blood cell counts of at least 4,000/mcL, platelet count of 150,000/mcL or more, blood urea nitrogen level of less than 30 mg/dL, and a creatinine concentration of less than 1.5 mg/dL. These patients did not have distant metastases as determined by routine preoperative staging (physical examination, chest X-ray, abdominopelvic computed tomography scan, or ultrasound of the abdomen), and patients with radiologically suspected serosal invasion signed informed consent forms explaining the potential advantages and disadvantages of EPIC. The available medical records of patients were reviewed, and the clinical and pathological stages were reclassified according to the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual. Among the 495 patients, 245 patients were included in this study, and 250 patients were excluded from the analysis. The patient cohort is presented in Fig. 1. The data for 245 patients were analyzed, and treatment-related complications were classified using the Clavien-Dindo system [9].
2. SurgeryThe type of gastrectomy performed, whether total or subtotal, was determined by the location and macroscopic type of gastric cancer. All of the patients underwent D2 or greater lymphadenectomy. Routine Roux-en-Y reconstructions with stapled esophagojejunostomy, stapled gastroduodenostomy, or hand-sewn gastrojejunostomy were carried out as reconstruction. In the event a surgeon found macroscopic serosal invasion of the gastric cancer intraoperatively, EPIC was performed only if the patient had signed the informed consent form preoperatively. In the EPIC group, before abdominal wall closure, three drain catheters were placed into both subphrenic spaces and Douglas’ pouch for drainage, and another catheter was placed into the lesser sac through the foramen of Winslow for both the administration and drainage of chemotherapeutic agents.
3. EPIC and adjuvant chemotherapyOn the day of the surgery, upon completion of reconstruction, the peritoneal cavity was irrigated with 37°C, 9% saline solution, until the fluid that was being drained from the catheters became clear. On the first postoperative day, one liter of 37°C, 0.9% saline solution, containing 10 mg/m2 of mitomycin C, was instilled rapidly into the peritoneal cavity via a catheter located in the lesser sac. All of the catheters were clamped for 23 hours. During the first six hours of treatment with mitomycin C, a urine output of greater than 1 mL/kg body weight/hour was maintained. On the second postoperative day, the peritoneal cavity was drained for one hour, and 700 mg/m2 of 5-fluorouracil (5-FU) plus 50 mEq of sodium bicarbonate in 1 L of 37°C 0.9% saline solution was instilled. The 5-FU instillations were repeated on a daily basis for a total of four administrations. The catheters were left in place until the drainage subsided. This protocol has been described elsewhere [6]. If the tumor penetrated the serosa or if lymph node metastasis was confirmed pathologically, adjuvant chemotherapy was recommended. 5-FU-based postoperative systemic chemotherapy regimens with cisplatin or epirubicin were administered to the patients who agreed to follow our recommendations regarding adjuvant systemic chemotherapy.
4. Classification of recurrence patternsThe pattern of recurrence was classified based on the findings of a physical examination, computed tomography, ultrasonography, or pathological examination. The presence of malignant ascites, of a rectal shelf, and nodular peritoneal thickening were classified as peritoneal recurrence. Liver metastasis, bone metastasis, and kidney metastasis were classified as hematogenous recurrence. If there was enlargement of the axillary, Virchow’s or para-aortic lymph nodes, or detection of malignant cells during the pathological examination of those lymph nodes, it was classified as nodal recurrence. Recurrence in the remnant stomach and stomach bed, and biliary obstruction were classified as loco-regional recurrence.
5. Statistical analysisThe proportions of patients with given characteristics were compared by a chi-square test or Fisher's exact test. Differences in the means of the continuous measurements were tested using a Student's t-test. The overall and diseasespecific survivals were calculated from the date of surgery until the death or at least the last follow-up, and Kaplan-Meier survival curves were plotted and compared using a log-rank test. A multivariate analysis was performed using the Cox proportional hazards model. Null hypotheses of no difference were rejected if the p-values were less than 0.05.
Results1. Clinicopathological characteristicsAmong the 245 patients included in this study, 65 patients received EPIC, while the remaining 180 patients did not. The characteristics of these patients are presented in Table 1. There were no significant differences between the two groups. Combined resection was defined as the resection of additional organs other than the spleen or the gallbladder.
2. Mortality and morbidityThe complication grade, as determined by using the Clavien-Dindo system, is presented in Table 2. There was no statistically significant difference between the groups (10.8% in the EPIC group vs. 8.9% in the non-EPIC group, p=0.804). All three grade I complications in the EPIC group were catheter-related problems, such as prolonged drainage, and were resolved by conservative treatment. All three grade I complications in the non-EPIC group were wound problems, such as seroma, or infections that were treated by conservative treatment or bedside drainage with wound care.
There was one grade II complication in each group. In the EPIC group, one minor anastomotic leak occurred, but resolved after fasting and total parenteral nutrition. There was one grade IIIa complication in the EPIC group, a peritoneal abscess, which was controlled by percutaneous drainage and the administration of antibiotics. In the non-EPIC group, more grade III complications occurred. There were six grade IIIa complications that were controlled by percutaneous drainage procedures, and three grade IIIb complications treated by reoperation under general anesthesia. There were no grade IV complications. There were two postoperative deaths (grade V) within 90 days of the surgery in the EPIC group, and three in the non-EPIC group; the difference was not significant (3.1% vs. 1.7%, p=0.611). In the EPIC group, two patients had transient leucopenia of higher than grade III, as classified by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0.
3. Survival analysisThere were 149 gastric cancer-related deaths. There were 29 cancer-related deaths (44.6%) in the EPIC group and 120 (66.7%) in the non-EPIC group. This difference was statistically significant (p=0.002). In univariate analyses of the clinical and pathological factors, the presence of pathological lymph node metastasis, extent of gastric resection, histological differentiation, and administration of EPIC had prognostic significance (Table 3). The 5-year overall and gastric cancer-specific survival rates of the EPIC group were 47.4% and 53.1%, respectively, and those of the non-EPIC group were 26.7% and 29.7%, respectively (p=0.012 and p=0.011). In a multivariate analysis with the factors that showed prognostic significance in univariate analyses (such as pathological lymph node metastasis, histological differentiation, extent of gastric resection, location of the primary tumor, and administration of EPIC), pathological lymph node metastasis, histological differentiation, extent of gastric resection, and administration of EPIC were found to be independent prognostic factors for the overall survival and gastric cancerspecific survival (Table 4).
4. Recurrence patternThe lower rate of recurrence in the EPIC group was significantly different from the rate of recurrence in the non-EPIC group, as described above. An analysis of the patterns of failure revealed a significantly lower rate of peritoneal dissemination after surgery with EPIC. Of the 149 patients whose modes of recurrence were identified, more peritoneal recurrences were observed in the non-EPIC group (32.2% vs. 18.5%, p=0.038). There were no statistically significant differences in the recurrence sites, except for peritoneum (Table 5).
DiscussionOne of the most common patterns of treatment failure in gastric cancer after potential curative surgery is peritoneal carcinomatosis, especially after gastric resection combined with extended lymph node dissection. Approximately half of the recurrences are peritoneal carcinomatoses [3,10]. The presence of intraperitoneal free tumor cells is a key factor in peritoneal recurrence. Free peritoneal tumor cells are often found in serosa-positive gastric cancer patients, and the viability of these cells is high [11]. It is assumed that tumor cells are shed into the peritoneal cavity from the tumor with serosal invasion [12]. Free peritoneal tumor cells are also postulated to be released by surgical procedures [13]. The detection of free tumor cells by peritoneal lavage cytology has been found to be associated with poor oncologic outcomes, and it is the most important factor that predicts peritoneal recurrence [14,15]. In a recent review analyzing 11 articles, Leake et al. [16] reported that the accuracy, sensitivity, and specificity of conventional peritoneal cytology predict peritoneal recurrence. The detection rate of tumor cells by peritoneal cytology increases with the serosal involvement of tumors [14,17]. However, the use of peritoneal cytology to make decisions regarding the use of immediate prophylactic measures to protect against peritoneal recurrence is limited by the poor sensitivity of the technique and the wide variations in the rate of positivity between institutions. De Manzoni et al. [18] noted that in gastric cancers with serosal invasion, the influence of peritoneal cytology on the survival rate was not significant. Efforts to increase the detection rate of intraperitoneal free tumor cells have been made using an immunocytochemistry, reverse transcription polymerase chain reaction, or the thin prep method. Positive peritoneal cytology is now regarded as an indication of M1 disease by the 7th edition of the AJCC staging system. Further, patients with positive peritoneal cytology are generally regarded as R1 resection even after potentially curative resection in accordance to the new Japanese Gastric Cancer Association (JGCA) classification.
At the time of enrollment, a dedicated cytologist was not available; thus, peritoneal washing cytology was not recommended. Therefore, our data cannot explain the effects of EPIC in patients with positive peritoneal washing cytology. Although the number of patients who received treatment was small, and despite the fact that peritoneal chemotherapy regimen was different from the one used in our protocol, a recent study by Imano et al. [4] revealed a significant different in the medial survival time.
The two major differences between IPC and systemic chemotherapy are the route of administration, and the timing of administration. The rationale for using the intraperitoneal route for chemotherapy administration is based on the high risk of peritoneal metastasis being a component of the first failure and the pharmacokinetics of intraperitoneal drugs [19,20]. After radical surgery for gastric cancer, tumor cells may either be completely resected or remained as a microscopic disease. The time of surgery is the point in time at which there is the smallest amount of the disease; thus, the time when adjuvant treatment can be most effective. In addition, free peritoneal tumor cells can escape from the contact with chemotherapeutics soon after surgery by the process known as ‘tumor cell entrapment’, which was proposed by Sugarbaker et al. [13]. Therefore, the most commonly used technique is to perform an intraperitoneal treatment (with or without hyperthermia) at the end of the gross resection. IPC is delivered in the operating room or recovery room or, at the very latest, within a few days from resection. The disappointing results of delayed IPC emphasize the importance of the timely administration of IPC [21].
Because the drains for EPIC are placed before the closure of the abdominal wall, a decision to administer EPIC should be made during the operation. The most accurate stage is the pathological stage, which is usually not available during surgery. Therefore, the intraoperative and clinical stages are important tools in deciding whether to administer EPIC. In this study, the authors’ decisions were based on the clinical stage, combined with the surgical stage. The prognostic significance of the clinical stage has been reported [22,23] Among patients with pathological stage II, IIIa, or IIIb, patients with an overestimated surgical stage had a significantly poorer survival rate than those with a nonoverestimated surgical stage [24].
Macroscopic serosal invasion, one of the most important risk factors for the peritoneal dissemination of gastric cancer, is a good criterion for the use of adjuvant IPC [23]. Patients with lymph node metastasis may benefit from IPC; however, it is difficult to predict which patients have positive nodes [23].
Therefore, the indication for EPIC used in this study was macroscopic serosal invasion. This is a simple and a reliable mode of indication. Our study found similar 5-year survival rates between patients with macroscopic serosal invasion (cT4a) and patients with pathological serosal invasion (pT4a); the gastric cancer-specific 5-year survival rates were 35.1% and 37.6%, respectively.
The treatment of gastric cancer patients with adjuvant systemic chemotherapy has not been satisfactory, except in the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) study [25]. A recent randomized clinical trial of adjuvant chemotherapy for high-risk, radically resected gastric cancer patients showed no benefit [1].
Yu et al. [23] reported that the use of EPIC to treat advanced gastric cancer was effective, especially in stage III patients. The 5-year survival rate for stage III patients in the EPIC group was 49.1%, and that of the non-EPIC group was 18.4% (p=0.011). In a retrospective study, Shi et al. [5] found that there was better survival in patients with adjuvant IPC. They reported a significant difference in the 5-year overall survival (IPC group 60.4% vs. non-IPC group 42.9%, p=0.001) [5], which was also verified in the present study; the 5 year overall survival rate in this study was 47.4% in the EPIC group and 26.7% in the non-EPIC group. Shi et al. [5] reported a significantly lower peritoneal recurrence rate in the IP (+) group compared to the IP (–) group (22.5% vs. 12%, p < 0.05). Although they reported similar results, there was a big difference from our study in the time of IPC administration. The median times for starting IPC in their protocol were 33 days, whereas we started EPIC at least within 24 hours post-operation.
Zhu et al. [8] reported decreased peritoneal recurrence in patients with hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin (34.9% vs. 10.2%, p=0.0128), and Zhu et al. [8] indicated that increased cytotoxicity of large volume antitumor drug against peritoneal free tumor cell was the cause of improving the survival rate.
A significant reduction in peritoneal recurrence (32.2% vs. 18.5%, p=0.038) was also observed in the present study.
The multivariate analysis revealed that the differentiation, pathological nodal metastasis, use of EPIC, and extent of gastric resection were independent prognostic factors. With respect to lymph node metastasis, N1 and N2 did not show a prognostic impact when compared with stage N0; however, N3 was found to be an independent poor prognostic factor for the enrolled patients. The patients who underwent total gastrectomy had a poorer prognosis than those who underwent subtotal gastrectomy. It has been reported that patients with proximal tumors have a poorer prognosis than patients with distal tumors. Our data indicate that the location of the tumor is a prognostic factor for gastric cancer-specific survival (p=0.036). The 5-year gastric cancer-specific survival rate of patients with proximal gastric cancer was 28.3%, whereas that of patients with distal gastric cancer was 43.3%. However, the reason for the poorer prognosis of patients with proximal gastric cancer is not fully understood. Patients in the EPIC group showed better survival and acceptable complications. The reason for the better survival might be attributed to the significantly reduced rate of peritoneal recurrence.
In this study, adjuvant chemotherapy did not show any survival benefit. However, the result is hard to be convinced since less than 30% of patients received adjuvant chemotherapy, and the efficacy of administered regimens had not been shown in those days. Therefore, a clinical trial comparing the prognostic impacts of adjuvant EPIC and adjuvant systemic chemotherapy with proven efficacy, such as S-1, should be conducted in a future study for verification.
Although there were limitations in this retrospective study, the use of EPIC as an adjuvant treatment for macroscopically serosa-invading gastric cancer was found to have the possibility of preventing peritoneal recurrence, thus enhancing survival.
ConclusionIn the present study, we found that the use of EPIC as an adjuvant treatment for gastric cancer with serosal invasion has the potential to improve survival, largely due to the reduced risk of peritoneal recurrence. Further clinical trials to prove the efficacy of EPIC in comparison to systemic chemotherapy should follow.
AcknowledgmentsThis research was partly supported by the promoting project for regional advanced components and materials (A000600028) of the Ministry of Knowledge Economy, Korea.
Fig. 1.Diagram of patients selection. HIPEC, hyperthermic intraperitoneal chemotherapy; IOIC, intraoperative normothermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy. 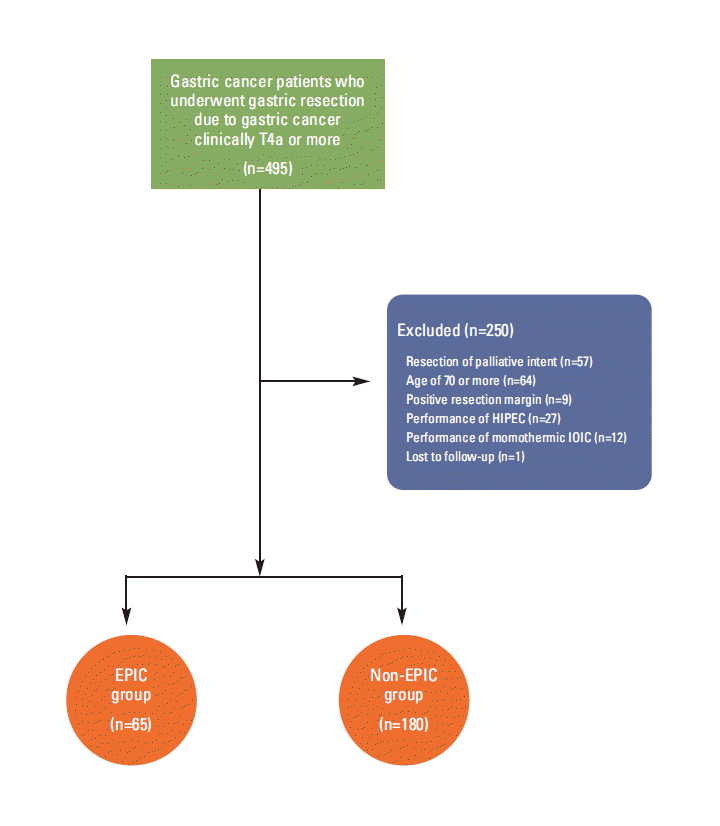
Table 1.Patient characteristics Table 2.Complication classification using the Clavien-Dindo system Table 3.Univariate survival analyses Table 4.Multivariate survival analysis for overall survival and gastric cancer-specific survival Table 5.Analysis of the modes of recurrence References1. Cascinu S, Labianca R, Barone C, Santoro A, Carnaghi C, Cassano A, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007;99:601–7.
2. De Vita F, Giuliani F, Orditura M, Maiello E, Galizia G, Di Martino N, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann Oncol. 2007;18:1354–8.
3. Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–7.
4. Imano M, Imamoto H, Itoh T, Satou T, Peng YF, Yasuda A, et al. Impact of intraperitoneal chemotherapy after gastrectomy with positive cytological findings in peritoneal washings. Eur Surg Res. 2011;47:254–9.
5. Shi C, Yang B, Chen Q, Yang J, Fan N. Retrospective analysis of adjuvant intraperitoneal chemotherapy effect prognosis of resectable gastric cancer. Oncology. 2011;80:289–95.
6. Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228:347–54.
7. Yu W. A review of adjuvant therapy for resected primary gastric cancer with an update on Taegu's phase III trial with intraperitoneal chemotherapy. Eur J Surg Oncol. 2006;32:655–60.
8. Zhu ZG, Tang R, Yan M, Chen J, Yang QM, Li C, et al. Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion: a long-term follow-up study. Dig Surg. 2006;23:93–102.
9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
10. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–42.
11. Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer. 1979;44:1476–80.
12. Ludeman L, Shepherd NA. Serosal involvement in gastrointestinal cancer: its assessment and significance. Histopathology. 2005;47:123–31.
13. Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21:233–48.
14. Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62.
15. Chuwa EW, Khin LW, Chan WH, Ong HS, Wong WK. Prognostic significance of peritoneal lavage cytology in gastric cancer in Singapore. Gastric Cancer. 2005;8:228–37.
16. Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S38–47.
17. Hayes N, Wayman J, Wadehra V, Scott DJ, Raimes SA, Griffin SM. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer. 1999;79:520–4.
18. de Manzoni G, Verlato G, Di Leo A, Tomezzoli A, Pedrazzani C, Pasini F, et al. Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg. 2006;30:579–84.
19. Shimada T, Nomura M, Yokogawa K, Endo Y, Sasaki T, Miyamoto K, et al. Pharmacokinetic advantage of intraperitoneal injection of docetaxel in the treatment for peritoneal dissemination of cancer in mice. J Pharm Pharmacol. 2005;57:177–81.
20. Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol. 2010;2:109–16.
21. Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol. 1994;12:970–4.
22. Park SR, Kim MJ, Ryu KW, Lee JH, Lee JS, Nam BH, et al. Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: a viewpoint in the era of preoperative treatment. Ann Surg. 2010;251:428–35.
23. Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985–90.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||