AbstractPurposeThe purpose of this study is to investigate the dosimetric and clinical influence of computed tomography–based (3-dimensional [3D]) simulation versus conventional 2-dimensional (2D)–based simulation in postoperative chemoradiotherapy (CRT) for patients with advanced gastric cancer in terms of parallel opposed anteroposterior-posteroanterior field arrangement.
Materials and MethodsA retrospective stage-matched cohort study was conducted in 158 patients treated with adjuvant CRT following curative surgery and D2 dissection from 2006 to 2008 at Samsung Medical Center: 98 patients in the 3D group; and 60 patients in the 2D group. For comparison of the dosimetric parameters between 3D plan and 2D plan, second sets of radiation treatment plans were generated according to the same target delineation method used in the 2D group for each patient in the 3D group (V2D). Acute toxicity, recurrence, and survival were analyzed. The median follow-up period was 28 months (range, 5 to 51 months).
ResultsThe 3D group showed better dose-volume histogram (DVH) profiles than the V2D group for all dosimetric parameters, including the kidneys, liver, spinal cord, duodenum, pancreas, and bowel. However, no difference in acute gastrointestinal toxicity and survival outcomes was observed between the 3D group and the 2D group.
ConclusionThe 3D plan enabled precise delineation of the target volume and organs at risk by visualization of geometric changes in the internal organs after surgery. The DVH of normal tissues in the 3D plan was superior to that of the V2D plan, but similar clinical features were observed between the 3D group and the 2D group.
IntroductionGastric cancer is one of the most common cancers in Korea [1], and the fourth most common cancer worldwide [2]. Although the proportion of early gastric cancer has increased as a result of routine screening in Korea and Japan, gastric cancer is often diagnosed in the advanced stage. Complete resection with adequate margins is widely regarded as the standard of care. However, even after curative surgery, locoregional recurrence and distant metastasis are significant problems, and the survival outcome is usually unsatisfactory. The Intergroup 0116 (INT 0116) study [3] demonstrated a major survival advantage by the use of adjuvant chemoradiotherapy (CRT) following curative surgery. The results of this study have changed the standard of care favoring the use of both chemotherapy and radiation therapy (RT) in the postoperative setting. RT is now used more commonly in treatment of gastric cancer.
Treatment planning guidelines recommended by the INT 0116 study suggested that parallel opposed anteroposterior-posteroanterior (AP-PA) fields represent a practical arrangement for the majority of patients [4,5]. With the wide availability of computerized treatment planning systems, the 3-dimensional (3D) plan has enabled more accurate delineation of high-risk target volumes, and usage of unconventional field arrangements for better dose distribution. However, computed tomography (CT)–based simulation was not mandatory in the past, and the majority of patients were treated based on the initial preoperative CT information, which did not reflect postoperative geometric changes in the internal organs. The internal organs were frequently relocated following surgery. An example of relocated kidneys delineated based on the preoperative CT (Fig. 1A) and the postoperative simulation CT (Fig. 1B) is shown in Fig. 1. The actual location of the kidneys after surgery was shifted upwards, and a considerable portion of the left kidney could have been exposed to radiation if the treatment plan had been made based on the preoperative CT alone. Therefore, we hypothesize that use of the CT-based (3D) RT plan, via improved and optimized radiation dose delivery and normal tissue sparing, would lead to decreased treatment-related toxicity and improved survival outcomes.
The 2-dimensional (2D) plan based on the preoperative CT was used until July 2007, and the 3D simulation was adopted thereafter at Samsung Medical Center. The goals of our study were two-fold. First, we compared the dosimetric parameters of AP-PA beam arrangement between 3D simulation and the conventional fluoroscopic 2D-based simulation plan. A few clinical studies have reported on differences in the dosimetric parameters of 3D AP-PA versus 2D AP-PA plans. Second, we evaluated the clinical impact of 3D versus 2D simulation in terms of acute toxicity, recurrence, and survival, by performing a matched cohort study.
Materials and Methods1. Patients’ characteristicsRetrospective analyses were performed in 321 patients treated with adjuvant CRT following curative surgery and D2 dissection from 2006 to 2008 at Samsung Medical Center. Basic eligibility criteria for postoperative adjuvant treatment were similar to those used in the INT 0116 trial except for the inclusion of patients with positive resection margins after surgery [3]. RT with 2D plan, which was in concordance with the INT 0116 protocol, was applied in 121 patients from January 2006 to May 2007 (2D group). RT with a CT-based treatment plan was applied in 200 patients from June 2007 to September 2008 (3D group): AP-PA beams were used in 98 patients; and 3-beam arrangements were used in 102 patients. After exclusion of 102 patients treated with the CT-based 3-beam plan, 98 patients treated with the CT-based AP-PA plan were compared with their 2D counterparts. For the purpose of objective comparability, stages were matched, and 158 patients were eligible for the analysis: 98 patients in the 3D group; and 60 patients in the 2D group.
A list of the patients’ demographic, treatment-related, and pathologic results is shown in Table 1. The median follow-up period was 24 months (range, 5 to 29 months) in the 3D group and 36 months (range, 5 to 51 months) in the 2D group. The median age was 53 years (range, 24 to 75 years), and male predominance was observed in both groups. The proportion of younger patients (≤ 50 years) was greater in the 3D group (36.7%) than in the 2D group (21.7%; p=0.041). No differences in the overall American Joint Committee on Cancer (AJCC) stage, T stage, and N stage were observed between the two groups. The 3D group included two patients with positive resection margins. A larger number of patients with close resection margins (less than 3 cm) was included in the 3D group than in the 2D group (47 and 23, respectively; p=0.024).
2. ChemotherapyTwo chemotherapy regimens were used during the current study period. The first regimen (IV fluorouracil and leucovorin [FL] regimen) consisted of intravenous fluorouracil (400 mg/m2/day) and intravenous leucovorin (20 mg/m2/day) for five consecutive days, starting at 3-7 weeks following surgery, followed by CRT beginning at four weeks after initiation of the first FL chemotherapy cycle. CRT consisted of a total radiation dose of 45 Gy over 5 weeks, with administration of intravenous fluorouracil (400 mg/m2/day) and leucovorin (20 mg/m2/day) on the first four and the last three days of RT. At 4 weeks following completion of CRT, two additional cycles of 5-day FL chemotherapy were administered at 4-week intervals.
The second chemotherapy regimen (oral capecitabine and cisplatin [CP] regimen) consisted of oral capecitabine (1,000 mg/m2, 2 times/day) for 14 consecutive days together with intravenous cisplatin (60 mg/m2/day) on the first day of each chemotherapy cycle spaced three weeks apart. After two cycles of CP chemotherapy, CRT, with the same RT dose schedule as in the FL regimen, was administered concurrently with oral capecitabine (825 mg/m2, 2 times/day) without intravenous cisplatin. At 3 weeks following completion of CRT, two additional cycles of CP chemotherapy were administered at 3-week intervals.
3. Radiation therapy1) 2D groupPreoperative CT information was used as a reference for the location of the normal organs before July 2007. RT simulation was performed in the supine position with the arms down. For identification of the anastomotic site and the duodenal stump, the suture line was identified on fluoroscopy with barium swallow. The superior margin of the field was set at the upper border of T11 vertebral body or the anastomotic site with at least a 2-cm margin. The inferior border was located at the junction of L2 and L3 vertebral bodies including the head of the pancreas. The right lateral margin was placed at the most lateral portion of the porta hepatis before the bifurcation of the right portal vein or at the duodenal stump or the head of the pancreas with a 1-cm margin. The left lateral border was placed at one vertebral body width from the left lateral border of the vertebrae (Fig. 2). A total dose of 45 Gy over 5 weeks in daily fractions of 1.8 Gy was prescribed at the mid-depth at the isocenter by manual calculation without tissue heterogeneity correction.
2) 3D groupRT plan policy was changed from 2D to 3D in July 2007. CT simulation was performed in the supine position with the arms down using IV contrast media. Radiation was targeted to the anastomotic site, duodenal stump, and regional lymph nodes. The tumor bed was included in the radiation target volume only in the case of T4 lesion. The anastomotic site, duodenal stump, major vessels (celiac trunk, splenic vessels, superior mesenteric vessels), regional lymphatics around the major vessels, and normal parenchymal organs (liver, kidneys) were contoured on each CT slice for delineation of the clinical target volume (CTV) and the organs at risk (OAR). The remnant stomach was not included in the target volume, according to previous studies by Lim et al. [6] and Nam et al [7]. The anastomotic site was excluded from the radiation field if the gross resection margin was 5 cm or greater. If the resection margin was less than 3 cm, the anastomotic site was included in the radiation field. If the resection margin was between 3 cm and 5 cm, inclusion of the anastomotic site was individualized considering the possible radiation toxicity to the adjacent normal structures. The same principle was applied to the duodenal stump as well. The upper, lower, and lateral margins were similar to those of the previous 2D plan, but individually modified based on the internal anatomic relationships. The irradiated nodal regions were individually modified based on the primary tumor location and the surgical findings. The definition of the Japanese Gastric Cancer Association [8] was used to delineate the regional lymph node areas. Number 7, 8, 9, 11, 12, 13, 14, and 16 lymph nodes were usually included. The parallel opposing oblique beam arrangements usually within ±15 degree range were also used to reduce the radiation dose to the kidneys. A total dose of 45 Gy over 5 weeks in daily fractions of 1.8 Gy was prescribed usually at the isocenter or mid-point of the CTV (Fig. 3).
3) RT plan comparisonThe planning CT scans for each patient in the 3D group were retrieved for evaluation. For comparison of the dosimetric parameters between the 3D and 2D plans, second sets of radiation treatment plans were generated according to the same method used in the 2D group for each patient in the 3D group (virtual 2D plan of 3D group, V2D). The corresponding dose-volume histograms (DVHs) of V2D plans were recorded for the kidneys, liver, spinal cord, duodenum, pancreas, and bowel. The spinal cord, duodenum, and bowel are the typical serial organs, and the mean dose and the maximal point doses were recorded for comparison. The percentage of the kidney volume receiving more than 15 Gy (V15) and 20 Gy (V20), and the percentage of the liver volume receiving more than 30 Gy (V30) are widely used in the literature for evaluation of renal and hepatic toxicities and were used for the current comparison.
4. Clinical outcomesAfter completion of the planned adjuvant treatment course, regular follow-up was performed, including a physical examination, complete blood cell count (CBC), liver function test, chest X-ray, abdomino-pelvic CT, and gastrofiberscopy. Follow-up intervals were 3 months for the first year, 6 months for the next 2 years and yearly thereafter.
Local recurrence was defined as recurrence at any of the anastomotic sites, duodenal stump, remnant stomach, and tumor bed. Regional recurrence was defined as recurrence in the regional lymph nodes within the radiation field. Distant metastasis was defined as recurrence outside the radiation field, including remote lymph node, peritoneal seeding, and other solid organ metastasis. If two or more failure sites developed at the same time, they were counted separately.
Complications were scored using the Radiation Therapy Oncology Group (RTOG) toxicity criteria. The mean values of 3D and V2D were assessed using the paired t test. Overall survival (OS) and disease-free survival (DFS) were estimated using the Kaplan-Meier method. The survival duration was defined as the time from surgery to the events or the last follow-up without events. Chi-square test, Cochran Mantel-Haenszel test, and Wilcoxon rank-sum test were used for comparison of differences in the prognostic factors, patterns of recurrence, and complication between the groups. Statistical significance was set at α=0.05. A p-value was based on two-sided tests.
Results1. Dosimetric impactTwo different RT plans derived from the same patient in the 3D group (3D vs. V2D) were compared. The median field width and length in the 3D group and V2D group was 10.0 cm (range, 8.0 to 15.0 cm) and 12.5 cm (range, 9.0 to 17.0 cm), and 13.0 cm (range, 11.0 to 15.0 cm) and 14.8 cm (range, 12.0 to 17.0 cm), respectively (p < 0.05), as shown in Table 2. A summary of the differences in DVHs between the two groups is shown in Table 3. All dosimetric parameters of normal tissues were lower in the 3D group, without exception.
2. Clinical outcomesThe OS rates were 95.7%, 89.5%, 82.2%, and 68.9% at 1, 2, 3, and 4 years, respectively. The DFS rates were 88.3%, 75.2%, 71.5%, 64.3% at 1, 2, 3, and 4 years, respectively. No difference in OS and DFS was observed between groups. No statistically significant differences in local, regional, and distant recurrence-free survival were observed between groups.
A list of patients with local or regional recurrences is shown in Table 4. Out of eight patients with local failure, six showed failure in the anastomotic site and two showed failure in the duodenal stump. Regional recurrence was observed in eight patients, four patients of the 3D group and four patients of the 2D group, respectively. All local or regional failures were either in-field or combined in- and out-field recurrences.
The prognostic factors for DFS and OS were evaluated by variables including age (≤ 50 years vs. > 50 years), sex, Eastern Cooperative Oncology Group (ECOG), T, N, stage, pathology, tumor grade, status of the resection margin, type of surgery, chemotherapy, and RT plan. In univariate analysis, ECOG 1 and 2 were poor prognostic factors compared to ECOG 0, advanced T, N and AJCC stage, positive or close resection margin, and type of surgery (subtotal gastrectomy with Billroth II reconstruction and total gastrectomy was worse than subtotal gastrectomy with Billroth I reconstruction) for DFS, distant recurrence-free survival, and OS, and close resection margin for regional recurrence-free survival. In multivariate analysis, ECOG, advanced T and N, and status of the resection margin were meaningful for OS.
The acute toxicity profiles of patients treated with FL regimen and those treated with CP regimen were analyzed separately. No difference was observed in both groups during and one month post-RT regardless of the chemotherapy regimen used, as shown in Table 5, whereas the between-group difference in the incidence of acute toxicity during RT with FL regimen was marginal (p=0.054).
Major complications, including grade 4 bowel toxicity were observed in two patients of the 2D group. Small bowel resection and anastomosis was performed in one patient with bowel perforation at six months post-adjuvant CRT and in the other patient with adhesive bowel obstruction at 14 months post-treatment.
Serial changes in CBC and blood chemistry of all patients were assessed at five time points before, during, and after RT: preoperative (baseline); postoperative (before the first chemotherapy); pre-RT; during RT; and after RT (at 3-4 weeks after completion of RT, just before initiation of the next chemotherapy cycle). No statistically significant differences in CBC and blood chemistry tests were observed between the two groups, as shown in Fig. 4.
DiscussionThis is the first study to quantify the dosimetric and clinical influences of the 3D plan with CT-based simulation in postoperative CRT for gastric cancer in terms of AP-PA field arrangement. The term first was used after conducting electronic searches on PubMed using the following keywords: stomach cancer, postoperative radiation therapy, CT simulation, AP-PA, and clinical outcomes. Theoretically, 3D-CRT enables more conformal dose delivery and normal tissue sparing than classic 2D RT. Several studies concerning radiation field arrangements in postoperative CRT for gastric cancer from the dosimetric aspects have been reported. For example, an Australian group suggested the ‘split-field’ technique, instead of the AP-PA plan, by dividing the planning target volume into two abutting sections, with each section treated using a separate, optimized field arrangement [9]. They reported lower mean doses to the right kidney (18 Gy vs. 35 Gy in 1/3 kidney, 6 Gy vs. 4 Gy in 2/3 kidney), the left kidney (18 Gy vs. 40 Gy in 1/3 kidney, 5 Gy vs. 5 Gy in 2/3 kidney), and the spinal cord (17 Gy vs. 45 Gy), and a higher dose to the liver (mean, 31 Gy vs. 10 Gy in 1/3 liver) in 15 studied patients. However, the problems of match-line, such as generation of cold or hot area, were unavoidable despite using a single isocenter technique. Another report by a group in Israel compared a non-coplanar four-field arrangement with the AP-PA plan and the four-field “box” technique in 19 patients [10]. According to the results, the doses to the spinal cord and both kidneys were significantly lowered by use of the non-coplanar four-field technique. Intensity-modulated RT (by either step-and-shoot or tomotherapy) is another treatment option under investigation, with more sparing of dose to the kidney and cord. However, it appears to offer only limited advantages when compared with sophisticated 3D conformal RT planning [11]. All of these studies were plan comparison studies using multiple beam arrangements with AP-PA plans without clinical results, which are different from the current study comparing AP-PA 2-beam arrangements contingent upon CT simulation and analyzing relevant clinical results in a larger number of patients. The gastric cancer consensus report [4] stated that “parallel opposed AP-PA fields are considered the most practical arrangement for the overwhelming majority of postoperative adjuvant radiotherapy cases.” In addition, the current National Comprehensive Cancer Network (NCCN) guidelines strongly recommend the use of CT simulation and 3D treatment plan. However, the CT-based simulation was not mandatory in the past and the majority of patients were treated based on the initial preoperative CT information, which did not reflect the postoperative anatomic changes. The gastric surgical adjuvant RT consensus report [4] recommended obtainment of three sets of simulation films for the traditional 2D treatment plan: the first set for identification of surgical clips and staple lines; the second set following administration of intravenous contrast for identification of the location of the kidneys; and the final set following barium swallow for identification of the anastomotic site, as well as the gastric stump. Before the CT era, the second set was not routinely obtained in practice following administration of intravenous contrast, and the first and third sets were usually used for the 2D simulation. The primary sites of the tumor, the major vasculature including the celiac axis, superior and inferior mesenteric arteries, porta hepatis, splenic artery, and both kidneys were identified based on preoperative CT images, without considering the probable postoperative position shifts. Only on rare occasions, when CT scan was performed postoperatively because of surgical problems, such as abdominal pain with fever, sustained ileus, suspicious abscess formation, etc., the postoperative CT scan could be used as the reference for the target delineation.
It is usually recommended that the doses to the surrounding organs should be kept as low as possible in order to reduce treatment-related side effects. In this study, for the purpose of comparing the dosimetric parameters between plans with or without CT-based simulation, second alternative RT plans, which followed the conventional 2D plan method with anatomic reference using the preoperative CT, were generated in the same patients. As expected, the CT-based simulation provided better DVH profiles than the traditional virtual simulation. Accurate visualization of the kidneys could be helpful in providing more appropriate renal shielding. All dosimetric parameters of the normal tissues were lower in patients of the 3D group, without exception. The kidneys, most notably, are sensitive to low dose radiation. Emami et al. [12] reported that the tolerance doses associated with a 5% risk of renal dysfunction at five years were 23 Gy after single, whole kidney irradiation and 20 Gy if both kidneys were irradiated. In addition, it is known that development of radiation-induced nephropathy could occur even at doses lower than the tolerance limit. Although there are reports of development of impaired renal function in children at 12 to 14 Gy [13], the threshold dose for renal damage in adults with normal baseline renal function is approximately 15 Gy with conventional fractionation. Renal damage may not manifest for years following RT, therefore, long-term follow-up is important. In one long-term study, the latent period was > 10 years in nearly half of the patients [14]. Therefore, the renal dose should be kept as low as possible through careful treatment planning, and CT simulation appears mandatory to achievement of this goal. In the current study, the mean dose to the left kidney was 767.4 cGy versus 1,734.2 cGy and that of the right kidney was 656.0 cGy versus 965.0 cGy for the 3D and V2D group favoring CT simulation, respectively (p < 0.001 for the left kidney, and p=0.004 for the right kidney). The mean dose to the spinal cord also favored the 3D group. Emami et al. [12] suggested 45 Gy as the cord tolerance dose. However, the maximal cord dose was 4,874.9 cGy in the V2D group, which was significantly higher than 4,737.5 cGy in the 3D group (p < 0.001). The mean dose to the liver was subclinical in all patients.
However, these improved dose profiles were not associated with better clinical outcomes of toxicity profiles, survival, and relapse patterns. There were no between-group differences in clinical features (all p > 0.05), whereas the difference in the incidence of acute toxicity during RT with FL regimen was marginal with a p-value of 0.054. The plan comparison was performed between the 3D group and the V2D group, a virtual 2D plan of the 3D group, not between the 3D group and the 2D group. In this respect, it should be considered that the dosimetric results of the V2D group may not reflect the actual clinical features of the 2D group as in the 3D group.
In summary, the 3D plan enabled precise delineation of the target volume and OAR by visualization of geometric changes in the internal organs after surgery. The DVH of normal tissues in the 3D plan was superior to that of the V2D plan; however, similar clinical features were observed between the 3D group and the 2D group.
ConclusionThe dosimetric parameters of normal tissues in the 3D AP-PA plan were lower than those of the V2D AP-PA plan, without exception, however, the clinical features, including acute toxicity, recurrence, and survival were similar between the 3D group and the 2D group. Our findings could facilitate understanding of the merits and demerits of CT-based AP-PA planning over 2D planning in postoperative treatment of patients with advanced gastric cancer.
Fig. 1.The anatomic relationship between the location of the kidneys and the radiation treatment field. The portion of the left kidney included in the radiation therapy (RT) field is highlighted in yellow color based on the preoperative computed tomography (CT) (A) and on the simulation CT (B) in the same patient. The actual location of the kidneys after surgery was shifted upward and a considerable portion of the left kidney was exposed to radiation during the actual treatment if the treatment plan had been made based on the preoperative CT. The portion of the right kidney included in the RT field is highlighted in green and no significant difference in terms of irradiated kidney volume was observed between the preoperative CT (A) and the simulation CT (B) in this patient. 
Fig. 3.Radiation field of the 3D group. (A) Axial plane with isodose curves and 2-beam arrangement in group 2A is shown. The white arrows represents 100% isodose line in red color. (B) Beam view of the anterior beam of the 3D group is shown here. 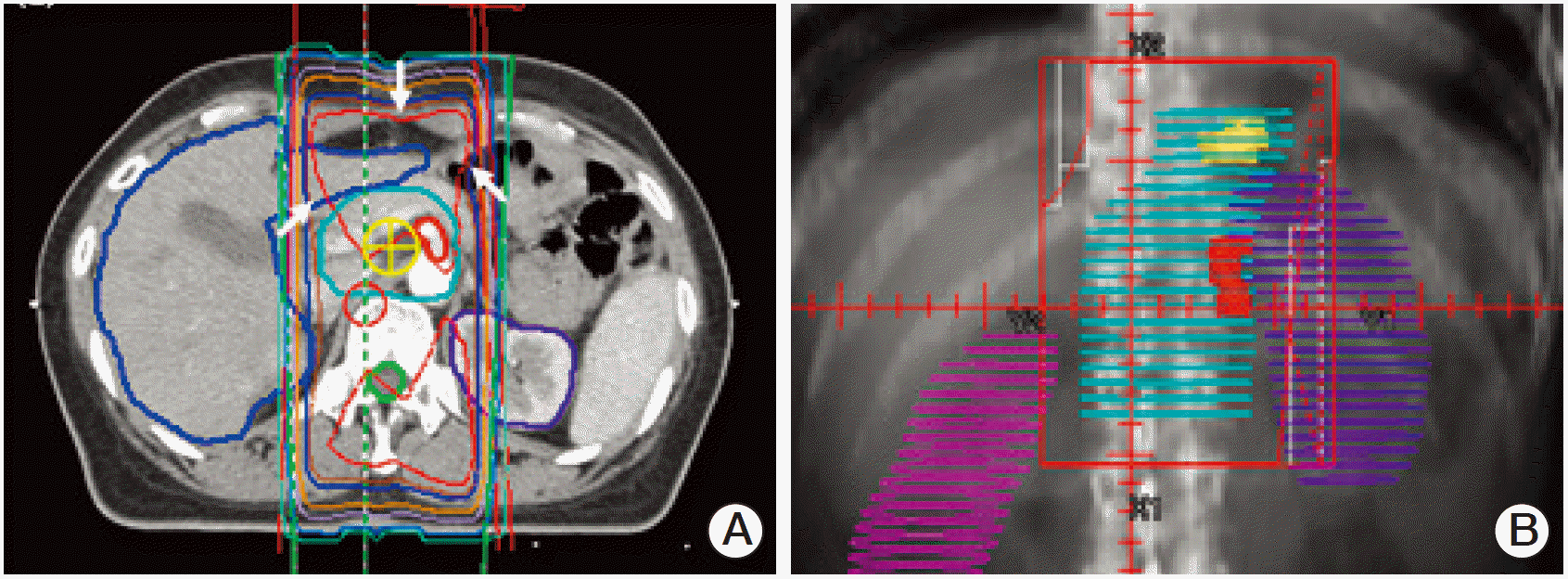
Fig. 4.Serial changes of complete blood cell count and blood chemistry of all patients at five time points before, during, and after radiation therapy (RT): preoperative (baseline); postoperative (before the first cycle of chemotherapy); pre-RT (right before the start of RT); during RT; and after RT (at 3-4 weeks after completion of RT and before the start of next chemotherapy). (A) Hemoglobin. (B) White blood cell count. (C) Absolute neutrophil count. (D) Aspartate aminotransferase. (E) Alanine aminotransferase. (F) Blood urea nitrogen. (G) Creatinine. Solid squares, open squares, and bars indicate 3D group, 2D group, and standard error, respectively. 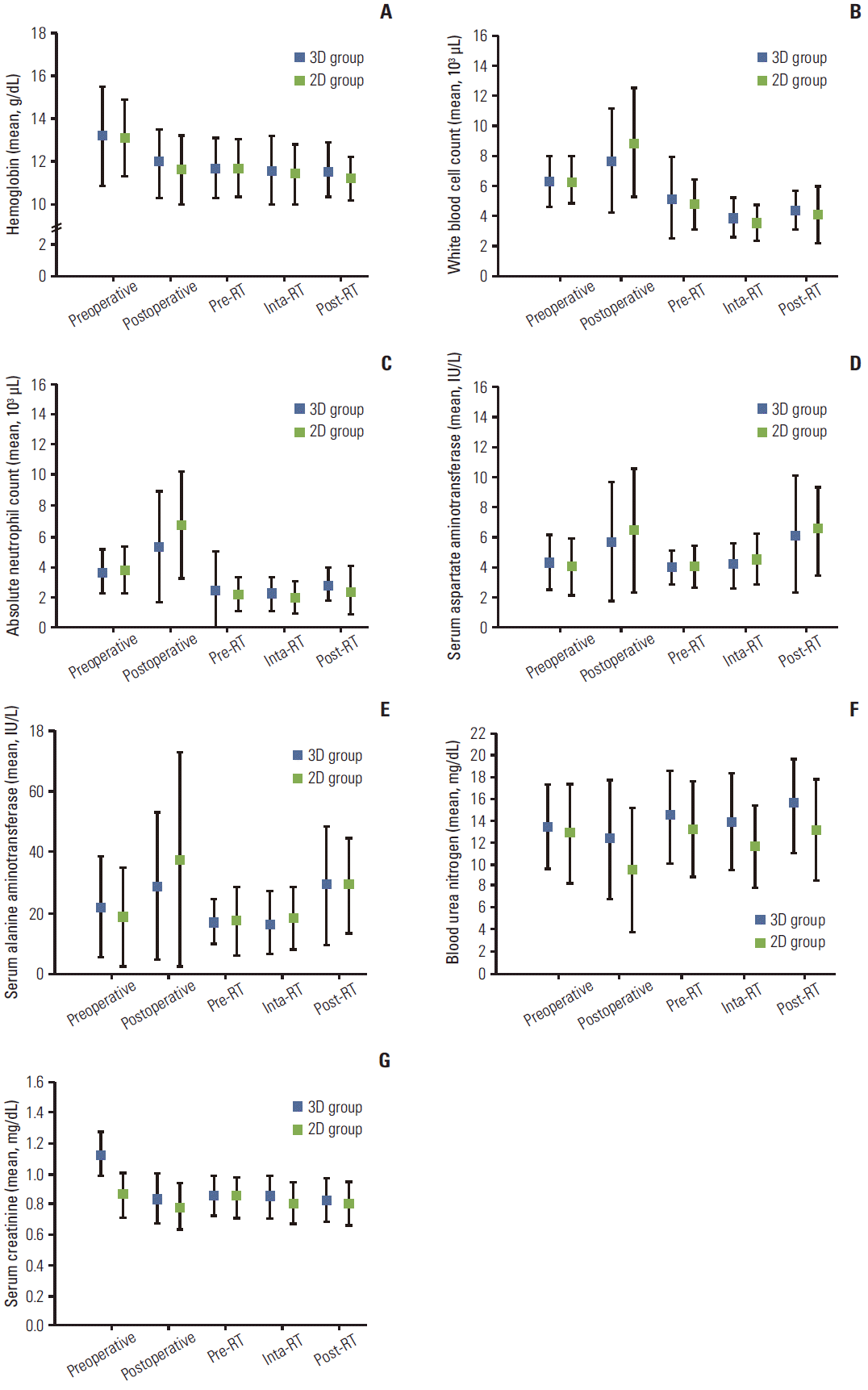
Table 1.Patients’ characteristics
Table 2.Field size difference of 3D group and V2D group
Table 3.Dosimetric comparison of 3D group and V2D group
Table 4.Patients with local and regional recurrences
Table 5.Acute GI toxicities by treatment group References1. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009;41:122–31.
2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
3. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
4. Smalley SR, Gunderson L, Tepper J, Martenson JA Jr, Minsky B, Willett C, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283–93.
5. Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002;12:187–95.
6. Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, et al. Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist's view. Br J Cancer. 2004;91:11–7.
7. Nam H, Lim DH, Kim S, Kang WK, Sohn TS, Noh JH, et al. A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys. 2008;71:448–55.
8. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24.
9. Leong T, Willis D, Joon DL, Condron S, Hui A, Ngan SY. 3D conformal radiotherapy for gastric cancer: results of a comparative planning study. Radiother Oncol. 2005;74:301–6.
10. Soyfer V, Corn BW, Melamud A, Alani S, Tempelhof H, Agai R, et al. Three-dimensional non-coplanar conformal radiotherapy yields better results than traditional beam arrangements for adjuvant treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:364–9.
11. Alani S, Soyfer V, Strauss N, Schifter D, Corn BW. Limited advantages of intensity-modulated radiotherapy over 3D conformal radiation therapy in the adjuvant management of gastric cancer. Int J Radiat Oncol Biol Phys. 2009;74:562–6.
12. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||