Chemotherapy in Patients Older than or Equal to 75 Years with Advanced Non-small Cell Lung Cancer
Article information
Abstract
Purpose
As the number of elderly patients diagnosed with non-small cell lung carcinoma (NSCLC) increases, the number of these patients receiving chemotherapy also increases. However, limited data exists regarding the use of chemotherapy in advanced NSCLC patients who are 75 years of age or older.
Materials and Methods
Between May 2002 and October 2008, data for 48 advanced NSCLC patients who were 75 years of age or older who had been treated with chemotherapy were retrospectively analyzed.
Results
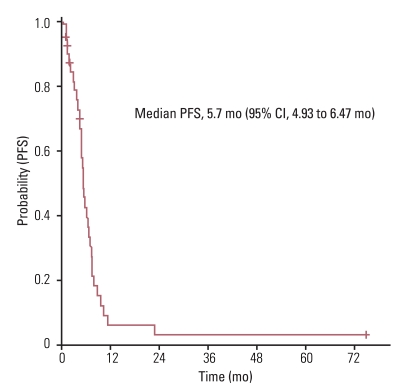
The median age of study participants at the time of first line chemotherapy was 76 years (range, 75 to 87 years) and their median Charlson comorbidity index was 2 (range, 1 to 4). Of the total 48 patients, 43 patients (90%) were treated by platinum-based doublet as a first line chemotherapy regimen. Median progression free survival for first line chemotherapy was 5.7 months (95% confidence interval [CI], 4.93 to 6.47 months) with an overall response rate of 33.3%. After first line chemotherapy, only 14 of the 48 patients (29.2%) received second line chemotherapy. The median overall survival (OS) for these patients was 8.2 months (95% CI, 4.44 to 11.96 months). Multivariate analysis results indicated that female gender and having received second-line or more chemotherapy were independent prognostic factors for increased OS for all 48 patients. Charlson Index was not a significant independent prognostic factor for survival. There were 9 treatment related deaths due to infectious causes (18.8%).
Conclusion
Patients 75 years of age or older with advanced NSCLC may obtain clinical benefit from the administration of platinum-based doublet or single agent chemotherapy. However, oncologists must consider the aspect of safety in relation to the clinical benefits when managing this patient group.
Introduction
Non-small cell lung carcinoma (NSCLC) accounts for 85% of all cases of lung cancer, which is increasingly a disease of older patients [1]. More than two thirds of NSCLC cases are diagnosed in persons of age 65 years or older and one in three patients is aged 75 years or older [2]. Data from the Surveillance, Epidemiology, and End Results (SEER) registry indicate that the median age at diagnosis of NSCLC patients is 69 years [3]. NSCLC can therefore be regarded as a disease of the elderly and the proportion of the elderly among NSCLC patients is expected to progressively increase due to the aging of the populations in many countries [4]. Aging is inextricably associated with physiological changes as they relate to functional status, organ function, and drug pharmacokinetics.
Aging is associated with decreases in marrow reserve, drug clearance and lean body mass. Furthermore, concomitant co-morbidities that affect functional status, general health and tumor symptoms are frequently present in this patient population [5]. Age itself is not a negative predictive factor and NSCLC treatment should not be omitted solely on the basis of chronological age [6]. It is functional impairment and comorbidity, not chronological age, that effect treatment tolerance and effectiveness in NSCLC cases [7].
Most elderly patients are diagnosed with an already advanced stage disease and the majority of those diagnosed with earlier stage disease will ultimately suffer disease progression [7]. Systemic chemotherapy has been a mainstay of therapy for patients with recurrent or advanced NSCLC. Several meta-analyses of randomized clinical trials have demonstrated that platinum-based combination chemotherapy induced a modest but significant survival advantage over best supportive care alone in patients with untreated, recurrent, or advanced NSCLC. However, platinum-based doublets are often unsuitable for elderly patients due to the existence of deficits in patient functional status and organ function. Oncologists have several treatment options for elderly patients with advanced NSCLC, including best supportive care without chemotherapy, single agent chemotherapy with a third generation drug, non-platinum-based combination chemotherapy, platinum-based combination chemotherapy, and new biologic agents. There has been limited available chemotherapy data for elderly patients with advanced NSCLC [8]. Elderly patients are excluded from participation in many clinical trials and often receive untested or inadequate treatment based on the long held, yet completely unsubstantiated, notion that cancer in the elderly is less aggressive and older patients are incapable of tolerating the exigencies of treatment [9]. Although the importance of more active treatment for elderly NSCLC patients is increasingly being recognized, the interactions between age, performance status and comorbidity, remain uncertain.
In this study, we analyzed the details of chemotherapy regimens and outcomes for NSCLC patients older than 75 years of age, a demographic group known to account for more than 30% of newly diagnosed NSCLC cases. In addition, we investigated performance status (PS) and comorbidity burden as a prognostic factor in those patients.
Materials and Methods
1. Patients
We retrospectively reviewed the records of 48 advanced NSCLC patients who were 75 years of age or older and who had been treated with systemic chemotherapy as a first line therapy at the Korea University Anam Hospital (Seoul, Korea) between May 2002 and October 2008. All patients had pathologically or cytologically proven advanced/metastatic or recurrent NSCLC. Clinicopathological information including gender, age at diagnosis, smoking history, and the type of chemotherapy were retrieved from the patients' electronic medical history records. We categorized patient comorbidity-burden according to the Charlson index [10,11]. The Charlson index provided a weighted index of comorbidity based on relative risks of death associated with 19 clinical conditions. We set cutoffs of Charlson index 0 to 1 as "mild," Charlson index 2 to 3 as "moderate," and Charlson index 4 or greater as "severe" comorbidities. This study was approved by the institutional review board at the Korea University Anam Hospital.
2. Chemotherapy
The chemotherapy regimen to be used was determined by the treating physician but the decision to administer first line chemotherapy depended, in all cases, on patient acceptance. Chemotherapy was repeated every 2-3 weeks according to the regimen. After every 2 or 3 chemotherapy cycles, tumor measurements were prepared using chest computed tomography scan and other tests used to stage the tumor. Responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0. Toxicity was graded according to the National Cancer Institute common toxicity criteria (NCI-CTC) version 3.0. The severity of any toxicities not defined in NCI-CTC were graded as 1=mild, 2=moderate, 3=severe or 4=very severe.
3. Statistical analysis
Treatment outcomes were estimated as response rate, disease control rate, overall survival (OS) and progression free survival (PFS). Tumor response was determined according to RECIST 1.0. The efficacy analysis was based on the intent-to-treat population. OS was defined as the time between the date of recurrent or metastatic disease diagnosis and the date of death from any cause. PFS was defined as the time between the date of the recurrent or metastatic disease diagnosis to the date of disease progression or death from any cause. The Kaplan-Meier method was employed to estimate the probability of survival and survival difference was analyzed by the log-rank test. The two-sided significance level was set at p<0.05. Cox proportional hazards regression model was employed in univariate and multivariate analyses to identify the significant independent prognostic factors of various clinical parameters for survival. Significant prognostic variables in the univariate analysis for OS were included in the multivariate analysis. p-value less than 0.05 was considered statistically significant.
Results
1. Patient characteristics
Of the 117 NSCLC patients 75 years of age or older, 48 were advanced NSCLC patients treated with systemic chemotherapy as a first line therapy. The clinical characteristics of these patients are shown Table 1. The median age of the patients was 76 years (range, 75 to 87 years) and the male/female ratio was 3.4/1.0. The median Eastern Cooperative Oncology Group (ECOG) PS was 1 (range, 0 to 2), and 11 patients (23%) had never smoked. At the time of diagnosis with NSCLC, 6 patients had diabetes mellitus and 11 patients were being treated for hypertension. The majority of patients had moderate comorbid disease with 2 patients having severe comorbid disease (Charlson index≥4). Twenty-nine of 48 patients (60.5%) had the histologic subtype of lung adenocarcinoma and all patients had advanced metastatic or recurrent disease.
2. Chemotherapy
As the first line of chemotherapy, 43 of 48 patients (90%) received platinum-based combination chemotherapy. For platinum-based doublet, the relative dose intensity was 83.2% and the median number of treatment cycles was four (range, 1 to 6 cycles). The chemotherapeutic agent added to platinum was gemcitabine in 28 patients, taxane in 10 patients, irinotecan in 4 patients and pemetrexed in 1 patient. Front line single agent chemotherapy including vinorelbine, gemcitabine and docetaxel, was administered to 5 patients (Table 2). Of the 48 patients receiving first line chemotherapy, 1 complete response and 15 partial responses were observed (overall response rate, 33.3%). Stable disease was observed in 18 patients (37.5%) and the disease control rate was 70.8%. Of the 48 patients with first line chemotherapy, progressive disease was observed in 5 patients (10.4%), and 14 patients (29.2%) received second line chemotherapy. The regimens used as second line therapy included epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in 6 patients, docetaxel in 4 patients, pemetrexed in 2 patients, and vinorelbine in 2 patients. There was no significant difference for OS results associated with second line chemotherapy regimens (p>0.005). Also, there was no significant difference in OS results between patients with or without EGFR-TKI as second or more line of therapy (p>0.005).
3. Survival and prognostic factors
All 48 patients were included in the survival analysis on an intent-to-treat basis. The median follow up duration was 9.7 months. Median PFS for first line chemotherapy was 5.7 months (95% confidence interval [CI], 4.93 to 6.47 months) (Fig. 1) and the median OS was 8.2 months (95% CI, 4.44 to 11.96 months) (Fig. 2). Univariate analysis revealed that decreased OS was significantly associated with male-gender, no-response for first line chemotherapy, and having not received a second line or more of chemotherapy (Table 3). From the multivariate analysis results, male-gender (hazard ratio [HR], 0.338; 95% CI, 0.141 to 0.808%; p=0.015) and not having received a second line or more of chemotherapy (HR, 0.357; 95% CI, 0.171 to 0.745%; p=0.006) were significantly associated with decreased OS (Table 3). Age (75-80 years vs. ≥80 years), ECOG PS (0-1 vs. 2-4) and Charlson index (mild comorbidity vs. moderate/severe comorbidity) were not significant independent prognostic factors for survival.
4. Toxicities and the causes of death
Toxicities observed during the treatments are listed in Table 4. NCI-CTC grade 3 or 4 hematologic toxicities included leukopenia in 26 patient, thrombocytopenia in 9 patients and anemia in 5 patients. Six patients experienced febrile neutropenia. Grade 3 or 4 non-hematologic toxicities included diarrhea, mucositis, peripheral neuropathy and anaphylaxis. The death of 47 patients was observed as revealed in Table 5. The majority of patients (76.6%) died due to the progression of NSCLC. One patient died of a cardiovascular event and another due to the progression of an underlying usual interstitial pneumonia, instead of NSCLC.
Discussion
Treatment of elderly patients presenting comorbidity with NSCLC is a serious challenge for clinicians. Generally, the elderly are excluded from participation in clinical trials and are undertreated, or they receive therapies that have not been tested in relevant clinical trials [12]. A systematic review of the recruitment barriers for elderly patients to clinical trials revealed exclusions related to trial design (e.g., protocol eligibility criteria with restrictions on age, comorbid conditions, or organ function requirements necessary to optimize treatment tolerability) and individual physician skepticism (e.g., the perception that the patient would not be able to tolerate treatment due to comorbidities and advanced age) [13]. However, several age specific subgroup analyses of phase III trials have failed to demonstrate differences in response rate, PFS and OS between age groups [14,15]. Little information is available regarding the management of advanced NSCLC in patients 75 years of age or older. In this study, median PFS for first line chemotherapy was 5.7 months (95% CI, 4.93 to 6.47 months) with an overall response rate of 33.3%. The median OS was 8.2 months (95% CI, 4.44 to 11.96 months). These outcomes were consistent with previous studies which were meta-analyses for first line chemotherapy in advanced or metastatic NSCLC [16,17]. Studies included in the meta-analyses were intended for patients in their 50s and 60s [16,17]. This finding implies that chronological age does not impact NSCLC treatment effectiveness. In this study, only 29.2% of patients received a second line of chemotherapy and only 6 received EGFR-TKIs as a line of treatment, due to limitations of the Korean medical insurance system. These factors may influence the relatively lower OS result observed.
Age is not a negative predictive factor and treatment should be not omitted on the basis of chronological age alone [6]. Oncologists increasingly recognize and accept the need for more aggressive treatment of elderly NSCLC patients. However, toxicity is an important factor influencing treatment decisions. Two retrospective pooled analyses reported similar time to progression and OS between older and younger patients, however, significantly higher toxic side-effects were reported for the older patients [18]. Nakamura et al., also revealed no differences in the response or survival rates between age groups (<70 years vs. ≥70 years) [19]. The most frequent toxicity, neutropenia, tended to be more common in the older patients. In this study, grade 3 or greater neutropenia was observed in 54.2% of cases and febrile neutropenia in 12.5%, a frequency comparable to that of other studies that were meta-analyses of platinum-based chemotherapy used in first line treatment of advanced NSCLC [16,17]. However, in this study, 9 patients died of infectious causes during first line chemotherapy. All of those patients had received cisplatin-based chemotherapy. These findings suggest that cisplatin-based chemotherapy may not be appropriate alone but could be used with platinum at doses attenuated to elderly NSCLC patients. Until now, the safety and efficacy of platinum-based combination chemotherapy in elderly patients with advanced NSCLC has not been confirmed. Although advanced age alone does not prelude combination therapies, given the increased likelihood of comorbidities, age should still be taken into consideration when selecting an appropriate regimen and dose in a clinical setting. However, there has been no data establishing appropriate chemotherapy regimens for elderly patients with advanced NSCLC.
Single agent chemotherapy has been considered the standard treatment for elderly NSCLC patients. A phase III multicenter trial demonstrated that single agent vinorelbine improved quality of life and survival compared with supportive care alone [20]. Several trials showed that the platinum-doublet may be more beneficial for elderly NSCLC patients than single agent chemotherapy [21]. Recently, Davidoff et al. [22], reported that, in clinical practice, two thirds of NSCLC patients with chemotherapy received a platinum-doublet first line regimen. In our results, 43 of 48 patients (90%) received a platinum-doublet first-line chemotherapy. Platinum-based chemotherapy is currently recommended as the standard approach for patients with advanced NSCLC [23,24]. However, to date, no prospective phase III trial that has explored the reproducibility of this benefit in elderly patients has been published. Due to association with significant organ toxicities, a rigorous risk versus benefit evaluation should be performed, particularly for elderly patients, before choosing cisplatin administration as an alternative to doublet.
Conclusion
This study had limitations including a small number of patients all coming from a single institution, and a heterogeneous patient group. Nevertheless, this study results suggest that advanced NSCLC patients, 75 years of age or older, may receive clinical benefit from platinum-based doublet or single agent chemotherapy. However, oncologists must consider the safety aspect as well as the clinical benefit when managing this patient group. The two important lessons from our study are: first, platinum-doublet chemotherapy could be effective for elderly patients with advanced NSCLC, and secondly, patient selection is important and a strategy should be explored to avoid fetal toxicity in this age group. Further specifically designed randomized phase III trials for elderly NSCLC patients are needed to evaluate the standard treatment against the various options which include targeted agent, single agent, non-platinum-doublet and platinum-doublet.
Notes
Conflict of interest relevant to this article was not reported.