Assessment of Tumor Regression by Consecutive Pelvic Magnetic Resonance Imaging and Dose Modification during High-Dose-Rate Brachytherapy for Carcinoma of the Uterine Cervix
Article information
Abstract
Purpose
To assess tumor regression, as determined by pelvic magnetic resonance imaging (MRI), and evaluate the efficacies and toxicities of the interim brachytherapy (BT) modification method, according to tumor regression during multi-fractionated high-dose-rate (HDR) BT for uterine cervical cancer.
Materials and Methods
Consecutive MRI studies were performed pre-radiotherapy (RT), pre-BT and during interfraction of BT (inter-BT) in 69 patients with cervical cancer. External beam radiotherapy (EBRT) was performed, using a 10 MV X-ray, in daily fraction of 1.8 Gy with 4-fields, 5 d/wk. Radiation was delivered up to 50.4 Gy, with midline shielding at around 30.6 Gy. Of all 69 patients, 50 received modified interim BT after checking the inter-BT MRI. The BT was delivered in two sessions; the first was composed of several 5 Gy fractions to point A, twice weekly, using three channel applicators. According to the three measured orthogonal diameters of the regressed tumor, based on inter-BT MR images, the initial BT plan was modified, with the second session consisting of a few fractions of less than 5 Gy to point A, using a cervical cylinder applicator.
Results
The numbers of patients in FIGO stages Ib, IIa, IIb and IIIb+IVa were 19 (27.5%), 18 (26.1%), 27 (39.2%) and 5 (7.2%), respectively. Our treatment characteristics were comparable to those from the literatures with respect to the biologically effective dose (BED) to point A, rectum and bladder as reference points. In the regression analysis a significant correlation was observed between tumor regression and the cumulative dose to point A on the follow-up MRI. Nearly 80% regression of the initial tumor volume occurred after 30.6 Gy of EBRT, and this increased to 90% after an additional 25 Gy in 5 fractions of BT, which corresponds to 73.6 Gy of cumulative BED10 to point A. The median total fraction number, and those at the first and second sessions of BT were 8 (5~10), 5 (3~7) and 3 (1~5), respectively. The median follow-up time was 53 months (range, 9~66 months). The 4-year disease-free survival rate of all patients was 86.8%. Six (8.7%) patients developed pelvic failures, but major late complications developed in only two (2.9%).
Conclusion
Our study shows that effective tumor control, equivalent survival and low rates of major complications can be achieved by modifying the fraction size during BT according to tumor regression, as determined by consecutive MR images. We recommend checking the follow-up MRI at a cumulative BED10 of around 65 Gy to point A, with the initial BT modified at a final booster BT session.
INTRODUCTION
The role of pelvic magnetic resonance imaging (MRI) has been widely evaluated during radiotherapy (RT) for uterine cervical cancer, with respect to diagnostic work-up, (1) RT planning (2~4), monitoring treatment response (5,6) and even for predicting the prognosis (7~9). Several authors have pointed out the anatomical changes that may occur due to tumor regression during external beam radiotherapy (EBRT) using consecutive MRI (6,10). Gong et al (6) reported the tumor regression rates using consecutive MRI studies during RT, and showed that significant changes in the tumor size can occur, even during the first few weeks of external radiotherapy, long before brachytherapy (BT). Huh et al (10) reported that consecutive MRI showed that the position of the uterus can change substantially during EBRT, and recommended that this should receive appropriate attention when planning 3D-conformal RT (3DCRT) or intensity modulated RT (IMRT). Currently, BT is usually delivered according to an initial plan, and without interim dose modifications. However, in practice, further tumor regression may occur during multi-fractionated high-dose-rate (HDR) BT, and this may result in anatomical changes. This implies that the initial isodose curves of the BT could result in over-coverage of the adjacent critical normal structures due to ongoing tumor shrinkage, in which case dose modifications to the initial BT schedule should be considered. However, few reports are available on tumor regression, as determined by consecutive MRI and interim dose modifications during multi-fractionated HDR-BT.
Recently, we performed consecutive MRI studies before radiotherapy (pre-RT), before brachytherapy (pre-BT) and during interfraction of BT (inter-BT), with the BT delivered in two sessions. The first BT session was planned by reviewing the pre-BT MR images, which consisted of several 5 Gy fractions to point A, twice weekly, using three channel applicators. If the gross tumor regressed markedly after several fractions, an additional inter-BT MRI study was undertaken, and according to the tumor regression observed, the second BT session, as a booster, was planned using fraction sizes smaller than 5 Gy, administered with a single channel cervical cylinder applicator. A cervical cylinder was used in the second BT session, as this type of applicator could be more easily applied, without the need for vaginal packing. We assessed the tumor regression and modified initial BT, as determined by follow-up MRI following variable fractions of the first BT session, and evaluated the efficacies and toxicities of this interim HDR-BT modification method, with respect to pelvic control, survival and complications, by performing a retrospective analysis.
MATERIALS AND METHODS
1) Patient population
Eighty-one patients with intact uterine cervical cancer, with FIGO stage IB1 through IVa, were treated by complete radiotherapy between December 1999 and July 2001. However, twelve patients received pre-RT pelvic MRI or pelvic CT only, without follow-up MRI during RT, and were excluded, leaving a total of 69 eligible patients. The eligibility criteria included a pathologically confirmed squamous cell carcinoma or adenocarcinoma of the cervix, and an Eastern Cooperative Oncology Group (ECOG) performance status index of 0~2. Pretreatment evaluation included complete medical history taking, physical examination, baseline blood tests, chest radiography, pelvic MRI (1.5-T system), and if necessary, sigmoidoscopy and cystoscopy. Patients were followed pre-RT, pre-BT and inter-BT using MRI. Informed consents were obtained from all patients, and the study protocol was approved by the institutional review board of Chonnam National University Hospital.
2) External beam radiotherapy (EBRT)
Before commencing brachytherapy, all patients underwent EBRT to the whole pelvis. EBRT was performed with 10 MV X-rays using the 4-field box technique, in daily fraction of 1.8 Gy, 5 days per week. EBRT, prior to midline shielding, was delivered up to around 30.6 Gy over 3.5 weeks. When the brachytherapy was started, further EBRT, with midline shielding, was delivered on the non-BT weekdays, up to total dose of 50.4 Gy, and if necessary, a booster dose of 5.4~9.0 Gy was delivered to the lateral pelvic wall.
3) Simulation of brachytherapy (BT)
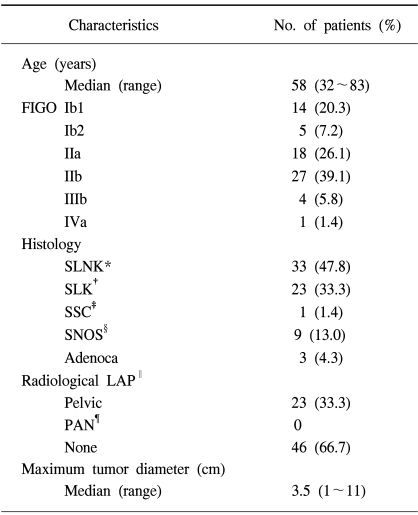
High-dose-rate brachytherapy was performed using a remotely controlled afterloading system (Varisource®, Varian, Palo Alto, CA), containing an radioisotope of 192Ir. The first BT session was performed using a Fletcher-Suit-Delclos three-channel system with a curved tandem and paired ovoids. Applicator insertion was performed on an outpatient basis, with non-narcotic analgesics. For BT simulation, orthogonal X-ray films of the anteroposterior and lateral views were taken with the applicators inserted. After application of several fractions of the first BT session, using the three-channel system, simulation of the second session was performed, using a single channel cervical cylinder applicator, after a follow-up MRI study. The cylinder was composed of acrylic material, and contained a central hollow slot to accommodate a tandem rod, through which 192Ir could be inserted into the uterus, cervix and vagina (Fig. 1). The cervical cylinders were 14 cm long, with diameters ranging from 2.0 to 4.0 cm, which were individually chosen according to the magnitude of the vaginal contracture. The length of the protruding tandem rod, which was either curved or straight, was inserted into the uterine canal and altered according to the size of the remnant tumor.
4) Follow-up MRI and brachytherapy planning
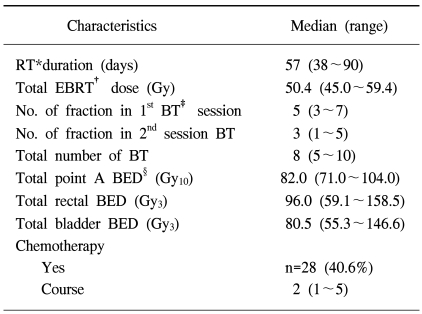
Before and during the BT, a follow-up MRI was undertaken to assess the tumor shrinkage. Follow-up MRIs were recommended at different times, according to initial tumor size. In patients with an initial maximum tumor diameter >4 cm, the follow-up MRI was performed just prior to the BT, as these patients were likely to show marked volumetric changes compared to those with smaller tumors. Planning for the first BT session was optimized by reviewing the pre-BT MR images, which demonstrated tumor regression due to the EBRT, with the resultant anatomical changes (Fig. 2). The first BT session was administered to cover the entire uterine cervix and body, with a 5 Gy fraction to point A, using three channel applicators, twice weekly, in several fractions. The position of the rectum, bladder and point A were defined according to ICRU 38 recommendation (11). Isodose curves were optimized by adjusting the dwell times of a 5 mm long stepping source at each position for the three applicators.

Initial bulky cervical tumor (Tm) and vaginal tampon (Tp) inserted into the upper vagina on a sagittal T1-weighted MRI prior to external beam radiotherapy (A), marked tumor volume regression on a follow-up MRI prior to BT (B) and small remnant tumor on a follow-up MRI during BT (C).
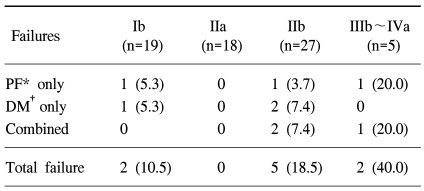
If the gross tumor regressed markedly after several fractions, an additional MRI study was undertaken, and this was followed by the second BT session, which was designed to cover only the regressed tumor portions, using a cervical cylinder. The location of point A was also defined according to ICRU 38 recommendations when using a cervical cylinder. The bladder point was defined as the most posterior part of the balloon, and the rectal point estimated to be at the anterior wall of a barium-filled rectum, on a line perpendicular to the central axis from the cervical external os (Fig. 3). We simply measured the three orthogonal diameters of the regressed tumor on the inter-BT MR images, and adjusted the dwell time via fitting of the 5 Gy isodose curves according to the magnitude of the measured volume on the anteroposterior and lateral simulation images. The resultant prescribed dose of point A was reduced to smaller fraction than 5 Gy (Fig. 3).

Isodose curves for the first BT session, administered using three channel applicators (A, B), and modified isodose curves for the second session, in which a cervical cylinder was used to treat the regressed tumor (C, D). A, B and R represent point A and the bladder and rectum reference points, respectively. The most inner curve represents a 5 Gy isodose and the outer curves show decrements of 1 Gy each.
In patients with an initial tumor diameter ≤4 cm, the first BT session was planned to cover the entire uterine cervix and body after reviewing the pre-RT MR images. This was also delivered using three channel applicators, with several 5 Gy fractions to point A. After the first session, a follow-up MRI was performed, and the tumor regression measured for planning of the second BT session, which was also delivered using a cervical cylinder, but with a reduced fraction size.
To perform a regression analysis on the relation between the percentage tumor volume reduction and the biologically effective dose to point A, the tumor volumes (Tvol) were measured by determining the three orthogonal diameters (x, y and z) from the MRI, according to the following equation:
Tvol = π/6×dX×dY×dZ
The biologically effective dose (BED) was calculated using a linear-quadratic model, with α/β ratios of 10 and 3 for tumors, and for the rectum and bladder, respectively.
5) Concurrent chemotherapy
Chemotherapy was recommended in patients with adequate hepatic, renal and cardiopulmonary functions, who were diagnosed as FIGO Ib-IIa with a bulky tumor (>4 cm) or radiological pelvic lymphadenopathy, and in all patients with a FIGO stage IIb or greater. The chemotherapy agents used were 5-FU (1 g/m2 body-surface area for day 1~4, by intravenous infusion) and cisplatin (50 mg/m2 on day 1, intravenously), which was repeated every 4 weeks up to four cycles.
6) Statistical methods
The endpoints of this study were the assessment of tumor regression during RT, survival, pelvic tumor control and late complications. The toxicity was assessed using the RTOG/EORTC late radiation morbidity scoring scheme (12). The tumor response was assessed immediately after the completion of radiotherapy. Patients were followed up every 3 months for the first 3 years, and then biannually. The overall survival was defined as the observed length of life from the start of RT to death, or to the last follow-up. Disease-specific survival was calculated from the start of RT to death due to disease, or to the last follow-up. Patients who developed central or pelvic relapses were considered as pelvic failures. Actuarial survival rates were calculated using the Kaplan-Meier method, and the differences between survival curves assessed using the log-rank test. The Statistical Package for the Social Sciences (SPSS ver.12.0) was used for the above analyses.
RESULTS
1) Patients and treatment characteristics
The median follow-up time was 53 months (range, 9~66 months) at the end of this study, as of May 31. 2005; follow-up was performed in all patients. The age range of the patients was 32~83 years (median, 58 years). The numbers of patients with FIGO stages Ib1, Ib2, IIa, IIb, IIIb and IVa were 14 (20.3%), 5 (7.2%), 18 (26.1%), 27 (39.1%), 4 (5.8%) and 1 (1.4%), respectively. Detailed patient characteristic data are listed in Table 1. Radiological lymphadenopathy (LAP) and the maximum tumor diameter were defined using MRI. Detailed data on the treatment characteristics are listed in Table 2. Chemotherapy was performed in 28 patients (40.6%), but the number of cycle was so variable that the effects of chemotherapy on the endpoints were not analyzed in this study. The radiotherapy characteristics were comparable to those from the literatures with respect to the biologically effective dose (BED) to point A, rectum and bladder as reference points. The median fraction number of the first and second BT sessions were 5 (3~7) and 3 (1~5), respectively. The resultant median total fraction of BT was 8 (5~10). The fraction size to point A for the first session was 5.0 Gy, with a median for the second session of 3.1 Gy (1.6~4.5).
2) Consecutive pelvic MRI and tumor regression during radiotherapy
A total of 146 MRI studies were performed, pre-RT, pre-BT and inter-BT (Fig. 4). The total number of patients receiving a pre-BT MRI was 27. These included all 19 patients with an initial tumor size >4 cm, and 8 with an initial tumor ≤4 cm, which all showed relatively less shrinkage macroscopically after EBRT. Of these 27 patients with a pre-BT MRI, 8 received an inter-BT MRI after the first BT session. All 42 patients with an initial tumor ≤4 cm received an inter-BT MRI. Of the 50 patients with an inter-BT MRI, 28 had an MRI after 5 fractions of the first BT session, 18 after 4 fractions, 3 after 3 fractions and 1 after 7 fractions.
A linear regression analysis was performed to identify any correlation between the cumulative BED10 to point A and the percentage of the tumor volume reduction; a statistically significant correlation was found (Fig. 5). The BED10 to point A was calculated each time for 77 follow-up MRI studies. According to the regression model, if the BED10 to point A was 36.1 Gy, equivalent to 30.6 Gy in 1.8 Gy fraction sizes of EBRT, about 78% of the initial volume regressed, and if the BED10 was 73.6 Gy, equivalent to 30.6 Gy by EBRT, plus 25 Gy in 5 fractions of BT, about 91% of the initial volume regressed. Twenty-eight patients showed early complete regression on follow-up MRI during radiotherapy, 7 of which showed complete regression, even though the BED10 to point A was <50 Gy. It seems necessary to check the follow-up MRI around 65 Gy of BED10 at the latest, which corresponds to 30 Gy of EBRT, plus 20 Gy, in four fractions of BT, and the interim BT modification should be considered according to the tumor regression.
3) Survival and failure patterns
The 4-year overall survival (OS) and disease-free survival (DFS) rates of the 69 patients were 76.7 and 86.8%, respectively. According to the FIGO stage, the 4-year OS of stages Ib, IIa, IIb and IIIb~IVa were 83.1, 94.4, 64.5 and 60.0% (p=0.10), and the corresponding 4-year DFSs were 89.5, 100, 80.7 and 60.0%, respectively (p=0.07, Fig. 6).
Sixty-five of the 69 patients (94.2%) achieved an initial complete response. Six patients (8.7%) developed pelvic failures as a component of failure (Table 3). According to the FIGO stage, the 4-year actuarial pelvic control rates of stages Ib, IIa, IIb and IIIb~IVa were 94.7, 100, 86.5 and 60.0%, respectively (p=0.02). Six patients had distant metastasis to the paraaortic nodes: 4 cases; lung: 3 cases; liver: 1 case; supraclavicular nodes: 1 case; and bone: 1 case.
4) Late toxicity
Grade 2 late rectal complications, according to the RTOG criteria, occurred in 2 patients (2.9%) and grade 2 bladder complications occurred in 2 patients (2.9%), but no patient developed grade 3 late complications. Two patients (2.9%) developed grade 4 late complications. Of these, one patient developed both a vesicovaginal and a rectovaginal fistula. The BEDs of this patient at point A, the rectum and bladder were 101 Gy10, 110.9 Gy3 and 146.6 Gy3, respectively, which were significantly higher than the median BEDs, 82 Gy10, 96 Gy3 and 80.5 Gy3, respectively, in all patients. The patient that developed fistulae showed an immediate partial response after radiotherapy, but died of a local recurrent disease at 32 months. Another patient had a grade 4 small bowel perforation and a BED at point A of 87 Gy10.
DISCUSSION
The role of pelvic MRI has been widely evaluated in the radiotherapy of uterine cervical cancer in terms of diagnostic work-up (1), RT planning (2~4), monitoring treatment response (5,6), and even in predicting the prognosis (7~9). Several authors have reported that consecutive MRI shows significant anatomical changes, even during the first few weeks of EBRT (5,6,10). Gong et al (6) reported on the determination of tumor regression rates during radiotherapy for cervical carcinomas using 4~8 consecutive MRIs, with an average time interval of 7 days. They reported that tumor regression began within a few days of commencing EBRT, with a mean tumor regression rate of about 7% per day. Hatano et al (5) followed the therapeutic effect of radiotherapy by MRI, and reported that tumor volumes decreased to 29% of their initial volume after 30 Gy of EBRT. In addition, these authors suggested that the reduction in tumor volume with 30 Gy could be used as a factor predictive of local control. Huh et al (10) reported on the interfractional variation, with respect to the uterus position, by comparing pre-RT and inter-RT MRIs, and showed that positional changes of the uterus during RT should be considered in the 3DCRT or IMRT planning of EBRT.
During multiple fractionated HDR-BT, it is known that the position of the BT applicators may change (13,14). In addition, further continuous tumor regression and resultant anatomical changes may also occur, especially during multi-fractionated HDR-BT. This implies that the initial BT isodose curves could result in over-coverage of the adjacent critical normal organs due to continued tumor shrinkage and; thus, interim BT modification should be considered. Current BT is usually planned from a subjective physical examination or with the use of pre-RT radiological images, and is delivered in accordance with the initial plan throughout the whole BT course. Most institutes in Korea perform BT by one-step planning, without interim modification, and use fraction sizes and numbers varying from 3 to 5 Gy and from 5 to 13, respectively (15).
MRI enables optimum evaluation of the soft tissue structures, tumor depiction and for direct measurements to be made in multiple planes. In particular, sagittal MRI provides helpful information on the uterus position and tumor volume for radiotherapy planning and delivery. It is known that MRI has an accuracy of up to 93% for assessment of the cervical tumor volume (16). Recently, a proposed guideline for image-based intracavitary BT for cervical carcinomas was reported by an image-guided BT working group (16). However, 3D-MRI planning is not yet ready for routine community practice, as MRI compatible applicators are expensive, and the constancy of organ fixation and patient immobilization for BT insertions (especially for multi-fractionated HDR-BT) are yet to be resolved. Our method of BT re-planning is relatively simple, involving determination of the regressed tumor diameters and modifying isodose curves by referring to the inter-BT MR images. The lengths and widths of the uterus and tumor were available on sagittal or coronal MR images, and thus the isodose curves were easily optimized using a cervical cylinder, with the doses to the adjacent organ reduced by constricting the isodose curves (Fig. 3). Cervical cylinders, with varying diameters and lengths, were easily applied without vaginal packing. In some institutions, cylindrical applicators have been designed for LDR-BT to replace the conventional ovoids, as the latter might deliver an insufficient dose to the ecto-cervix in a narrowing vaginal apex or to mid to lower vaginal lesions, and because they require no vaginal packing (17,18). Often, some patients complain of pain during outpatient based HDR-BT, despite analgesic sedation when the conventional two ovoids are inserted into a vagina constricted by EBRT or old age, especially in Korea. Furthermore, it is also troublesome to use gauze packing for every insertion of multi-fractionated HDR. In our study, the cylindrical applicator used eliminated the need for vaginal packing and facilitated easier application during the second BT.
Our results concur with the literature in terms of survivals and failure patterns, with very low rates of major late complications. The present study showed that nearly 80% of the initial tumor volume regresses after about 30.6 Gy of EBRT, which implies that marked anatomical changes occur before the implementation of BT (Fig. 5). Thus, when planning BT, pre-BT MRI, rather than a subjective physical examination, is helpful in terms of achieving appropriate tumor coverage, whilst sparing adjacent organs - especially in patients with large initial tumors. Our study also showed that further tumor volume reduction can occur during BT. After the BED10 at point A reaches 73.6 Gy, equivalent to a cumulative dose of 30.6 Gy of EBRT combined with 25 Gy of BT in 5 fractions, 91% reduction in the tumor volume was observed (Fig. 5). Thus, the inter-BT MRI findings can be used to plan the second BT session to ensure adequate tumor coverage and spare normal organs; moreover, the availability of such MRI findings is mandatory in patients that have not received a pre-BT MRI. Some authors have reported that early tumor regression rates on follow-up MRI after EBRT, may predict the likelihood of local tumor control (5,19). Mayr et al (19) reported that local control was achieved in 94% of those showing tumor volume regressions of >50% of the initial volume after 20 Gy of EBRT. Hatano et al (5) found that if a tumor regressed by >70% after 30 Gy of EBRT that local control was eventually achieved in 100% of subjects. Our study also showed that local control was achieved in all 15 patients, who showed a median regression volume of 75.4% after 30.6 Gy of EBRT. Conversely, of the six patients that developed pelvic failure, four showed a lesser reduction in the tumor regression than predicted by our linear regression model (Fig. 5).
Many studies have reported on the acceptable BED at reference points. Lee et al (20) reported the results of BT using a 3 Gy fraction size, and recommended that the rectal BED3 should be kept below 130 Gy. Clark et al (21) found a dose response relationship and threshold for late rectal complications above 125 Gy3 at the rectal reference point. Ferrigno et al (22) reported higher 5-year late bladder complication rates among patients treated with a BED of >125 Gy3 at the bladder reference point, although the differences were not statistically significant. Fu et al (23) reviewed the literature results of HDR brachytherapy treatment, with reported major complication rates ranging from 0.3 to 10%. Our study showed that only two patients (2.9%) developed major late complications. The rectal and bladder BED3 of one patient, with both a rectovaginal and a vesicovaginal fistula, were 110.9 and 146.6 Gy, respectively, which were significantly higher than the median doses administered to all patients (96 Gy and 80.5 Gy, respectively). Another patient had an emergency operation due to small bowel perforation 6 months after the initiation of radiotherapy. She had received a total of 50.4 Gy of EBRT to the whole pelvis, with midline shielding at 25.2 Gy, and was further boosted with 5.4 Gy to the parametrium with one cycle of concurrent chemotherapy. We now perform EBRT up to 45 Gy to the whole pelvis, including midline shielding, in cases receiving concurrent chemoradiotherapy. In this study, twenty-eight patients (40.6%) received chemotherapy, but the number of cycle was variable, so the effect of chemotherapy on outcomes was not analyzed in this study. Further studies, in more homogenous patient populations, following conditioning stage, tumor size, use of chemotherapy, and the balance between EBRT and BT, will be required.
CONCLUSIONS
Our results show that effective tumor control, equivalent survival and low rates of major complications can be achieved by modifying the fraction size during BT according to tumor regression, as determined by consecutive MR imagings. Our study showed that 80% of tumor volume regression occurs after 30.6 Gy of EBRT and 90% after the subsequent addition of 25 Gy of BT in 5 fractions. We recommend checking the follow-up MRI around 65 Gy of the BED10 at the latest, which corresponds to 30 Gy of EBRT plus 20 Gy in four fractions of BT, and the interim BT modification should be considered according to the tumor regression.
Notes
This was supported by the Chonnam University Hospital Research Institute of Clinical Medicine (Grant no. CUHRI-Y-200405).