GASTric Cancer HER2 Re-Assessment Study 2 (GASTHER2): HER2 Re-assessment for Initially HER2-Negative Advanced Gastric Cancer Patients after Progression on First-Line Treatment
Article information
Abstract
Purpose
Heterogeneous human epidermal growth factor receptor 2 (HER2) overexpression in gastric cancer may lead to a misdiagnosis of HER2 status. Accurate assessment of HER2 status is essential for optimal treatment as novel HER2-directed agents are being investigated in various clinical settings. We evaluated the usefulness of HER2 re-assessment following progression on first-line treatment in initially HER2-negative advanced gastric cancer (AGC) patients.
Materials and Methods
We enrolled 177 patients with baseline HER2-negative AGC and performed HER2 re-assessment after progression on first-line treatment from February 2012 to June 2016 at Asan Medical Center, Seoul, Korea. The re-assessed HER2 status was analyzed with baseline HER2 status and clinical characteristics.
Results
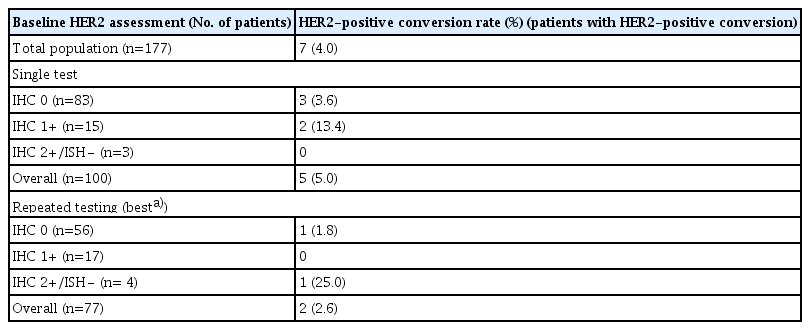
The median age was 54 years (range, 24 to 80 years), and 123 patients (69.5%) were men. Seven patients (4.0%) were HER2-positive on the re-assessment. Patients with baseline HER2 negativity confirmed by a single test (n=100) had a higher HER2-positive re-assessment rate compared to those who had repeated baseline testing (n=77) (5.0% vs. 2.6%). Among the patients with single baseline HER2 testing, the rate was higher in patients with baseline HER2 immunohistochemistry (IHC) 1+ compared to those with IHC 0 (13.4% vs. 3.6%).
Conclusion
Overall, 4.0% of patients with baseline HER2-negative AGC were HER2-positive on re-assessment, and the HER2-positive re-assessment rate was higher among patients who had a single test at baseline. HER2 re assessment may be considered for initially HER2-negative patients to determine their eligibility for HER2-directed therapy, particularly if their HER2 negativity was determined by a single test, especially if they had a single baseline HER2 IHC 1+ test.
Introduction
With a million new cases diagnosed annually around the world, gastric cancer is one of the most common causes of cancer-related death [1]. Systemic chemotherapy improves survival outcomes compared to the best supportive care alone for patients with metastatic or recurrent advanced gastric cancer (AGC), although the prognosis is still dismal [2]. With advances in our knowledge of the molecular biology of gastric cancer, several targeted agents have been studied for the treatment of AGC. Trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2), was the first therapeutic agent proven effective for patients with HER2-positive AGC [3]. The pivotal phase 3 ToGA trial showed a significant survival benefit of first-line trastuzumab-based combination chemotherapy compared to chemotherapy alone, and combined therapy is currently the standard of care for HER2-positive metastatic AGC patients [4]. Testing for HER2 status is recommended for all patients with metastatic or recurrent AGC who are planning to receive systemic treatment [5].
HER2 status in gastric cancer is assessed by HER2 immunohistochemistry (IHC) and in situ hybridization (ISH) analysis, and HER2 IHC 3+, or IHC 2+ with HER2 amplification confirmed by ISH, are defined as HER2-positive [5]. Previous studies reported a HER2-positive rate from 17% to 22.1% among gastric cancer patients [6,7]. Unlike breast cancer, gastric cancer often shows intratumoral HER2 heterogeneity and discordance of HER2 overexpression between primary and metastatic lesions within the same patient, and heterogeneous HER2 positivity may result in false-negative testing results for HER2 status among patients with AGC [3]. Variability in HER2 staining, defined as 30% or less HER2 IHC-positive cells, was found in 50.3% of all randomized patients in an exploratory analysis of the phase 3 ToGA trial [7]. In an observational study, only 34 among 87 patients (39%) with HER2-positive gastric cancer showed homogeneous HER2 overexpression by IHC analysis [8]. To this end, at least five or more endoscopic biopsy specimens and ideally six to eight specimens are recommended for testing HER2 overexpression in newly diagnosed AGC patients to avoid false-negative results [9,10]. Moreover, our previous GASTHER1 study showed that repeated assessment of HER2 status in initially HER2-negative patients resulted in 8.7% being declared HER2-positive prior to first-line treatment after repeated endoscopic biopsy and 5.7% after repeated biopsy of metastatic lesions [11].
In real-world practice, repeated biopsy for HER2 assessment at baseline for patients with initially HER2-negative AGC is not routinely performed and most patients have a single test conducted for HER2 positivity at baseline. Moreover, even after repeated assessments prior to first-line treatment, we may miss HER2-positive patients due to the heterogeneity of HER2 positivity in gastric cancer. Indeed, re-assessment of HER2 status after progression on first-line treatment may change the status of patients with HER2 false-negative results at baseline and make them eligible for HER2-directed treatments if they are positive on the re-assessment. The purpose of the current GASTric cancer HER2 re-assessment study 2 (GASTHER 2) was to evaluate the usefulness of HER2 re-assessment after first-line treatment without HER2 targeted therapy for AGC patients who were initially HER2-negative.
Materials and Methods
1. Participants
Between February 2012 and June 2016, a total of 177 patients with histologically confirmed HER2-negative locally advanced, recurrent, or initially metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma who progressed on first-line chemotherapy and had a re-assessment of HER2 after progression at the Asan Medical Center, Seoul, Korea were included in the analysis (Fig. 1). Clinical data were extracted from the electronic medical record system, including demographics, baseline clinical characteristics, and treatment outcomes. This study protocol was performed according to the guidelines established by the Declaration of Helsinki. Ethics approval of the study protocol was provided by the Institutional Review Board (IRB No. 2022-0993), and all patients provided written informed consent before enrollment.
2. HER2 evaluation
HER2 IHC was performed with archived formalin-fixed paraffin-embedded tissue of the biopsy specimen and stained for HER2 with anti HER2/neu (4B5) rabbit monoclonal primary antibody (Ventana Medical System, Tucson, AZ) using a Benchmark automatic immunostaining device (Ventana Medical System). The expression level of HER2 was scaled from 0 to 3+ according to the gastric cancer consensus guidelines [10]. ISH was performed in cases of IHC 2+, and results with a HER2/CEP17 ratio ≥ 2 or an average HER2 copy number ≥ 6.0 signals/cell were considered HER2 amplification. HER2 positivity was defined as IHC 3+ or IHC 2+ with HER2 amplification confirmed by ISH.
All patients had a baseline assessment of HER2 performed prior to first-line chemotherapy with a biopsy specimen or a surgical specimen. Some of the patients had repeated HER2 testing with different biopsy specimens at baseline per the previous GASTHER1 study results [11]. We divided the baseline HER2 negativity into two groups according to whether HER2 negativity was confirmed by single or repeated testing. Patients were treated with first-line systemic chemotherapy that did not contain trastuzumab or other investigational drugs targeting HER2. After progression on first-line treatment, the patients underwent another biopsy and were re-assessed for HER2 status.
3. Statistical analysis
Baseline and re-assessed HER2 status and clinical outcomes of second-line treatment for patients with HER2-positive status on re-assessment were analyzed with descriptive methods. We also compared their clinical characteristics according to HER2 status on re-assessment. A chi-square test or Fisher’s exact test was applied for comparisons of categorical variables as appropriate. Survival outcomes were estimated by the Kaplan-Meier method and were compared using the log-rank test. Progression-free survival (PFS) was defined as the time from initiating first-line treatment to disease progression by Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 or death from any cause, whichever came first. Overall survival (OS) was defined as the time from initiating first-line treatment to death from any cause. Survival outcomes of the second-line treatment (PFS2 and OS2) were also analyzed for patients who were HER2-positive on re-assessment. All statistical analysis was performed with R ver. 4.1.2 [12] (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value of less than 0.05 was considered statistically significant.
Results
1. Patient characteristics
The median age of the patients was 54 years (range, 24 to 80 years), with 40 (22.6%) older than 60 years, and 122 (68.9%) were men (Table 1). Only four patients (2.4%) had primary GEJ cancer, while 161 patients (97.6%) had primary gastric cancer, and 128 patients (73.1%) had poorly differentiated adenocarcinoma. Fluoropyrimidine plus platinum doublet regimens were given as first-line treatment to 159 patients (89.8%). The other 18 patients (10.2%) were treated with various regimens, including 5-fluorouracil and leucovorin plus irinotecan, fluoropyrimidine monotherapy (S-1, 5-fluorouracil, and TAS-118), and other investigational drugs. The median PFS and OS of the first-line treatment were 6.12 months (95% confidence interval [CI], 5.56 to 7.07) and 13.8 months (95% CI, 11.9 to 16.1), respectively.
2. Baseline HER2 status
Baseline HER2 negativity in 77 patients (43.5%) was confirmed by repeated HER2 testing, and 100 patients (56.5%) underwent a single test (Table 1). Among 100 patients with a single HER2 test, 83 patients (83.0%) were IHC 0, 15 patients (15.0%) were IHC 1+, and two patients (2.0%) were IHC 2+/ISH negative at baseline. For the evaluation of HER2 status among patients with one test, endoscopic biopsy specimens were used for 82 patients (82.0%), and a surgical specimen from the primary tumor or biopsy specimens from the metastatic lesion was used for nine patients (9.0%) each. Among the 82 patients who underwent endoscopic biopsy, 69 patients had information about the number of biopsy pieces obtained during the endoscopic biopsy, and 54 patients (78.3%) had 5 or more collected.
As for the patients who underwent repeated testing at baseline (n=77), 56 patients (72.7%) were IHC 0, 17 (22.1%) were IHC 1+ as their best result out of the repeated tests, and 4 (5.2%) were IHC 2+/ISH negative as their best result (Table 1). At the initial baseline testing, 65 patients (84.4%) underwent endoscopic biopsy, and 21 out of 22 patients with information about the number of biopsy pieces collected (95.5%) had five or more. At repeated baseline testing, 54 patients (70.1%) underwent endoscopic biopsy, and 42 out of 51 patients with information about the number of biopsy pieces collected (82.4%) had five or more obtained from the biopsy.
3. Re-assessment of HER2 status after first-line treatment
Overall, seven patients (4.0%) with baseline HER2 negativity were HER2-positive on re-assessment with the biopsy specimen obtained after progression on first-line treatment. Among the patients with a baseline HER2-negative status confirmed by a single test, five patients (5.0%) were HER2-positive on re-assessment, whereas two patients (2.6%) among those who had repeated testing at baseline were HER2-positive on re-assessment (Table 2). Among patients who had a single test for baseline HER2 assessment, patients with baseline HER2 IHC 1+ had a higher HER2-positive re-assessment rate (13.4%, 2 patients out of 15) compared to those with IHC 0 (3.6%, 3 patients out of 83). Among patients who had repeated testing at baseline, one patient with baseline HER2 IHC 0 was HER2-positive on re-assessment (1.8%, 1 patient out of 56). None of the patients with IHC 1+ from repeated baseline testing (n=17) and one patient (25.0%, 1 patient out of 4) with baseline HER2 IHC 2+ and ISH (−) was HER2-positive on re-assessment. A detailed comparison of the baseline and re-assessed HER2 status is provided in S1 Table (single test at baseline) and S2 Table (repeated testing at baseline).
4. Clinical characteristics of patients according to re-assessed HER2 status
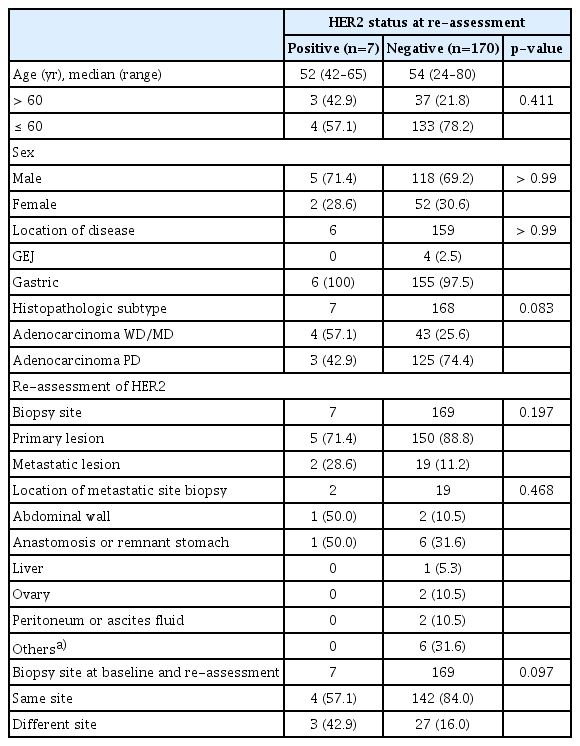
Although those who were HER2-positive on re-assessment tended to have a higher proportion of patients with well-differentiated or moderately differentiated tumors (57.1%) than those who were not (57.1% vs. 25.6%, p=0.083) (Table 3), there was no significant difference in clinical and pathologic characteristics according to re-assessed HER2 status. Among 155 patients who had re-assessment with re-biopsy of the primary tumor, five patients (3.2%) were HER2-positive on re-assessment, and two patients (9.5%) among 21 patients with re-biopsy of the metastatic lesion were re-assessed as HER2-positive. Patients who had HER2-positivity on re-assessment had a higher proportion of patients who had re-biopsy at a different site than at the baseline assessment (42.9%, 3 out of 7 patients) compared to those with HER2-negative status at the re-assessment (16.0%, 27 out of 169 patients, p=0.097).
5. Second-line treatment for patients with HER2 positivity on re-assessment
Among the seven patients with HER2 positivity on re-assessment, two patients received HER2-targeted treatment as their second-line therapy: patient 2 received trastuzumab plus docetaxel, but the clinical outcomes could not be assessed because the patient was lost to follow-up after two cycles. Patient 4 was included in the GATSBY trial, which compared trastuzumab emtansine (T-DM1) with taxane for previously treated HER2-positive tumors [13], and received (T-DM1) as second-line treatment (Table 4). Patient 4 had a baseline HER2 IHC score of 0 by a single test on the resected gastric cancer specimen (Fig. 2A). A re-biopsy of the abdominal wall metastatic lesion and re-assessment of HER2 testing revealed a HER2 IHC score of 3+ (Fig. 2B) after progression on the first-line treatment (vorinostat plus capecitabine and cisplatin). The patient showed PR as the best response, with a PFS of 9.47 months for second-line treatment (Fig. 2C).
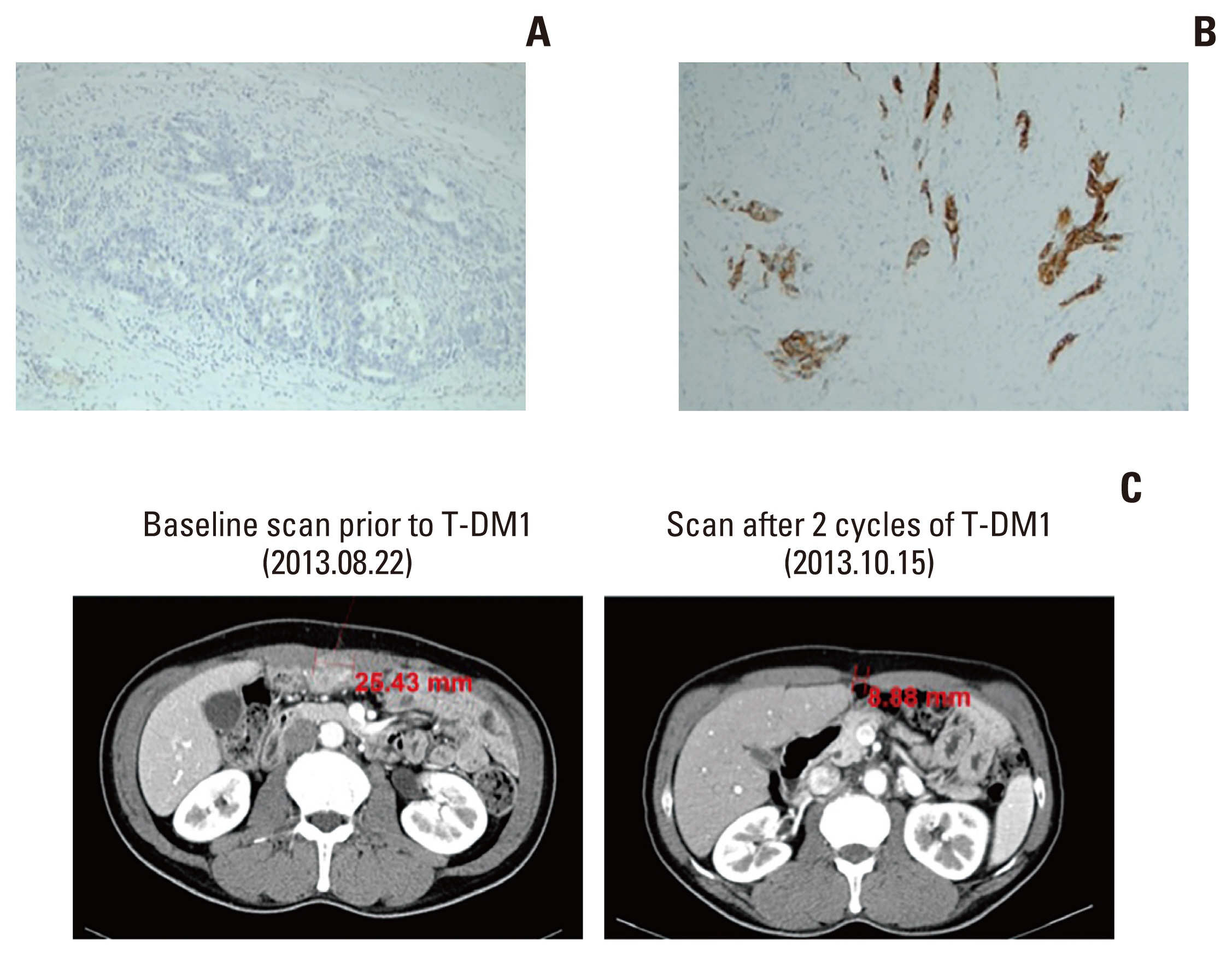
Human epidermal growth factor receptor 2 (HER2) immunohistochemistry staining results at baseline and after progression on first-line treatment, and the response to second-line treatment with T-DM1. (A) Baseline HER2 immunohistochemistry of a resected gastric adenocarcinoma specimen showing no staining (×100). (B) HER2 immunohistochemistry of a biopsy specimen from an abdominal wall metastasis after progression on first-line treatment showing an intensity of 3+ (×100). (C) Representative abdominopelvic computed tomography scan showing a decrease in the diameter of the abdominal wall metastasis from 25.43 mm at baseline (left) to 8.88 mm (right) after two cycles of T-DM1.
Discussion
This study reports the rate of patients testing positive for HER2 upon re-assessment of a new biopsy specimen after first-line treatment among patients who tested HER2-negative initially. Overall, 4.0% of patients showed HER2-positive status on re-assessment, and the HER2-positive re-assessment rate was higher among patients with single HER2 testing at baseline (5.0%) compared to those who had repeated testing (2.6%). Notably, patients with HER2 IHC 1+ at baseline had a higher HER2-positive re-assessment rate (13.4%) than those with HER2 IHC0 at baseline (3.6%) among patients with single testing. Our results suggest that, albeit for a small proportion, HER2 re-assessment should be considered for patients with baseline HER2 negativity to determine if they are eligible for HER2-directed agents for subsequent treatment. While the previous GASTHER1 study demonstrated the clinical significance of repeated biopsy for patients with initial HER2 negativity at baseline, the current GASTHER2 study showed the clinical significance of HER2 re-assessment even after progression on first-line treatment [11]. As repeated HER2 testing at baseline is not routinely performed, our study suggests the usefulness of HER2 re-assessment after first-line treatment and underscores the importance of HER2 re-evaluation for gastric cancer.
Patients with HER2-positive status on re-assessment who had a single test done at baseline in the current study may be considered as initially HER2 false-negative. Clonal selection of HER2 overexpressing tumor cells may explain the HER2 positivity on re-assessment following first-line treatment, although the concept of clonal selection of HER2 overexpressing cancer cells after non HER2 targeted systemic chemotherapy in gastric cancer remains theoretical. Conversely, there were several previous studies suggesting clonal selection of tumor cells that do not overexpress HER2 after HER2-targeted treatment [14–16]. From our previous GASTHER3 study, 48 patients with HER2-positive AGC given a trastuzumab-based treatment had a matched HER2 assessment with pre- and post-treatment biopsy specimens, and 14 patients (29.1%) showed loss of HER2 positivity [16]. Since patients who had HER2 re-assessment with re-biopsy of a metastatic lesion showed a higher positivity rate than those with re-biopsy of the primary lesion (9.5% vs. 3.2%), HER2 re-assessment with a metastatic lesion may be preferred. However, biopsy of a metastatic site is not always possible, and the majority of patients with re-assessed HER2 positivity had re-biopsy of the primary lesion (5 out of 7). Also, in the GASTHER1 study, the HER2 positivity rate by repeated endoscopic biopsy was 8.7%, while it was 5.7% for metastatic or recurrent site biopsy [11].
One patient among the seven patients with HER2 positivity on re-assessment (baseline HER2 IHC 0 vs. re-assessed HER2 IHC 3+) received T-DM1 as second-line treatment and showed PR as the best response to T-DM1 and a PFS2 of 9.47 months. The survival outcome of this patient was numerically longer than the median PFS of 4.4 months (95% CI, 4.2 to 5.3 months) of ramucirumab plus paclitaxel from the phase 3 RAINBOW trial result [17], which would have been used for the treatment of this patient if a re-assessment of HER2 had not been performed. PFS2 of this patient from the initiation of T-DM1 was longer than the median PFS of 2.7 months (95% CI, 2.8 to 4.0 months) shown in the GATSBY study [13]. The OS2 was 22.78 months, which is also longer compared to the results of the prespecified biomarker analysis of the GATSBY trial, showing an OS of a median 9.5 months (95% CI, 8.0 to 11.7 months) among patients with IHC 3+ [18]. This patient may have shown a better survival outcome as he was naïve to anti-HER2 agents compared to GATSBY trial patients who received trastuzumab-based first-line treatment, which may be associated with increased resistance to anti-HER2 agents in the second line. Our results may imply a clinical benefit of HER2-targeted agents among patients who are HER2-positive on re-assessment after first-line treatment without a HER2-directed agent. Indeed, re-assessment of HER2 after progression on first-line treatment may provide patients who are anti HER2 naïve with better treatment options.
Nevertheless, our findings should be cautiously interpreted because this was only a single case and not all patients with HER2 positivity on re-assessment after first-line treatment may respond to second-line treatment with HER2-targeted agents. The association between HER2 heterogeneity and poor outcomes of trastuzumab based treatment was also evaluated in several prior studies, and those with heterogeneous expression showed inferior survival outcomes compared to those with homogeneous overexpression [8,19,20]. HER2 positivity on re-assessment would be strongly associated with heterogeneity of HER2 positivity and may be associated with a poor response to HER2-directed therapy. In a recent observational study investigating the outcomes of first-line trastuzumab-based treatment for patients with HER2-positive AGC, the survival outcomes were significantly poor among patients with HER2 positivity only after repeated testing compared to those who were HER2-positive on their first test in terms of both PFS (log-rank, p=0.017) and OS (log-rank, p=0.036) [21]. It may also be challenging to consider the re-assessment of HER2 status in all patients with initially HER2-negative results considering the small proportion of patients with HER2 positivity on re-assessment and the risk of complications from re-biopsy.
On the other hand, a recent phase 2 clinical trial showed the efficacy of trastuzumab deruxtecan (T-Dxd) in patients with prior trastuzumab treatment, who may have HER2 heterogeneous expression or even loss of HER2 positivity by clonal selection of non-HER2 overexpressing tumor cells [14–16,22]. The bystander killing effect of well-diffused cleaved payloads may explain the effect of T-Dxd, and its preclinical efficacy has been proven in low HER2 overexpressing tumors [23,24]. Currently, there are no recommended second-line treatment options for re-assessed HER2-positive patients with progression on first-line fluoropyrimidine plus platinum, which is the standard first-line treatment for HER2-negative patients. In a phase 2 clinical trial of trastuzumab plus paclitaxel for previously treated metastatic HER2-positive AGC patients who were naïve to anti-HER2 agents, the median PFS was 4.53 months for patients who progressed on a platinum-based regimen (61% of the total population), although this should be cautiously interpreted since we do not know whether the patients in this trial were HER2-positive initially or on re-assessment [25]. T-Dxd may be beneficial for HER2-targeted agent naïve patients with HER2 positivity on re-assessment after first-line treatment, and re-assessment of HER2 status after first-line treatment may provide patients with better treatment options. Moreover, re-biopsy with additional molecular studies may identify patients who are eligible for targeted therapies other than HER2, including claudin 18.2 and FGFR2b [3,26,27].
Our study has several limitations. Our current report is an observational study from a single center. Only a single patient’s outcome in response to HER2-targeted therapy who had HER2-positive status on re-assessment is described.
In conclusion, we found that 4% of initially HER2-negative AGC patients were HER2-positive on re-assessment with a new biopsy after progression on first-line treatment. The HER2-positive re-assessment rate was higher in patients with baseline HER2 negativity confirmed by a single test, especially among patients with HER2 IHC 1+ at baseline. Re-assessment of HER2 following first-line treatment may be considered in initially HER2-negative patients to determine their eligibility for HER2-directed therapy, particularly for patients who had a single test for HER2 at baseline, especially if they had a baseline HER2 IHC 1+ result.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
All procedures in studies involving human participants were performed in accordance with the ethical standards of the Institutional Review Board of Asan Medical Center and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (IRB approval No. 2022-0993), and all patients provided written informed consent before enrollment.
Author Contributions
Conceived and designed the analysis: Kang YK.
Collected the data: Kim HD, Ryu MH, Park YS, Moon M, Kang YK.
Contributed data or analysis tools: Hyung J, Kim HD, Ryu MH, Park YS, Kang YK.
Performed the analysis: Hyung J, Kim HD, Ryu MH, Kang YK.
Wrote the paper: Hyung J, Kim HD, Ryu MH, Park YS, Kang YK.
Conflicts of Interest
Conflicts of interest Nothing directly related to this work. Out of this work, YKK has served as a consultant for Liscure, ALX Oncology, Zymeworks, Amgen, Novartis, Macrogenics, Daehwa, Blueprint, Surface Oncology, BMS, and Merck (MSD). MHR received an honorarium and served on the advisory boards of Ono Pharmaceutical, BMS, MSD, Lily, Taiho, Novartis, Daiichi Sankyo, and AstraZeneca, and served as a consultant for DAEHWA Pharmaceutical, BMS, Lily, and Ono Pharmaceutical.