Efficacy of Prophylactic Cranial Irradiation According to the Risk of Extracranial Recurrence in Limited-Stage Small Cell Lung Cancer
Article information
Abstract
Purpose
We aimed to evaluate the effectiveness of prophylactic cranial irradiation (PCI) for “early brain metastasis”, which occurs before extracranial recurrence (ECR), and “late brain metastasis”, which occurs after ECR, in limited-stage small cell lung cancer (LS-SCLC).
Materials and Methods
We retrospectively analyzed 271 LS-SCLC patients who underwent definitive chemoradiation. All patients were initially staged with brain magnetic resonance imaging and positron emission tomography. Intracranial recurrence (ICR), ECR, progression-free rate (PFR), and overall survival (OS) were analyzed as clinical endpoints. The competing risk of the first recurrence with ICR (ICRfirst) was evaluated. Significantly associated variables in multivariate analysis of ECR were considered as ECR risk factors. Patients were stratified according to the number of ECR risk factors.
Results
The application of PCI was associated with higher PFR (p=0.008) and OS (p=0.045). However, PCI was not associated with any of the clinical endpoints in multivariate analysis. The competing risk of ICRfirst was significantly decreased with the application of PCI (hazard ratio, 0.476; 95% confidence interval, 0.243 to 0.931; p=0.030). Stage III disease, sequential, and stable disease after thoracic radiation were selected as ECR risk factors. For patients without these risk factors, the application of PCI was significantly associated with increased OS (p=0.048) and a decreased risk of ICRfirst (p=0.026).
Conclusion
PCI may play a role in preventing early brain metastasis rather than late brain metastasis after ECR, suggesting that only patients with a low risk of ECR may currently benefit from PCI.
Introduction
Small cell lung cancer (SCLC) is a clinically aggressive type of lung cancer. According to the Korean registry, 13.6% of newly diagnosed lung cancer cases are SCLC [1]. Even with a high responsiveness to chemotherapy and radiation therapy (RT), SCLC tends to metastasize to the brain, which is a significant obstacle to long-term survival [2]. Prophylactic cranial irradiation (PCI) has been performed for patients with SCLC who showed a response after treatment to reduce brain metastasis. Whether PCI is beneficial for better clinical outcomes has been a constant topic of discussion. For limited-stage (LS)–SCLC, the current evidence is mainly based on a meta-analysis by Auperin et al. [3]. As more than 20 years have passed, there have been several changes in clinical practice after the publication of the meta-analysis. Advancements in imaging modalities such as positron emission tomography (PET) and magnetic resonance imaging (MRI) have been incorporated into the staging of SCLC. Brain MRI performed for neurologically asymptomatic SCLC patients revealed brain metastasis in 15% of them [4]. Clinicians have become unsure of the effectiveness of PCI for LS-SCLC patients staged with brain MRI. There have been new findings from trials on extensive-stage (ES)–SCLC. In a previous randomized trial by Slotman et al. [5], which proceeded without brain MRI staging, PCI was effective in reducing brain metastasis and improving survival. However, a recent randomized trial by Takahashi et al. [6] used brain MRI for staging and surveillance, and PCI did not improve overall survival (OS). The findings from ES-SCLC trials have contributed to doubts about the effectiveness of PCI for LS-SCLC.
Although clinical guidelines still recommend PCI for LS-SCLC in various clinical situations [7,8], retrospective series with modern imaging modalities have shown conflicting results regarding the effectiveness of PCI in decreasing brain metastasis and improving survival [9,10]. Therefore, although there may be some subsets of LS-SCLC patients who can benefit from PCI, it may not be beneficial for others. Several studies have attempted to distinguish such subgroups [11,12]. However, there is no current consensus on the clinical situation appropriate for PCI. We hypothesized that the effect of PCI on LS-SCLC patients might differ between “early brain metastasis”, which occurs before extracranial recurrence (ECR), and “late brain metastasis”, which occurs after ECR. Based on this hypothesis, progressed extracranial disease after thoracic treatment may metastasize to the brain even with initial intracranial disease control, and this type of brain metastasis may not be preventable with PCI. The purpose of this study was to evaluate the effectiveness of PCI for early and late brain metastasis in LS-SCLC and to stratify the clinical outcomes of PCI according to the risk of ECR.
Materials and Methods
1. Study population
The medical records of patients who underwent definitive chemoradiation (concurrent or sequential) for LS-SCLC at two institutions between January 2004 and December 2017 were reviewed retrospectively. All patients analyzed in this study underwent staging procedures, including brain MRI and PET, and the absence of brain metastasis was confirmed at the time of initial diagnosis. Patients who experienced progressive disease based on Response Evaluation Criteria in Solid Tumors (RECIST) after definitive treatment were excluded as they were not suitable for PCI. Patients who underwent stereotactic ablative radiotherapy for the primary thoracic disease were also excluded. Therefore, 271 patients were eligible for this study.
2. Treatment and follow-up
The standard procedures of treatment for LS-SCLC were 6 cycles of etoposide plus cisplatin chemotherapy or etoposide plus carboplatin chemotherapy and concurrent RT. RT was recommended with the 3rd cycle of chemotherapy or earlier. Procedural details, such as the number of chemotherapy cycles or the start date of RT, were often adjusted based on disease response and treatment adherence. The standard dose-fractionation scheme of thoracic RT was 50 to 60 Gy in 25 to 30 fractions. Treatment was delivered once a day. A hypofractionated regimen (dose per fraction > 2 Gy) was also permitted. Either three-dimensional conformal RT or intensity-modulated RT were used in RT planning and delivery. Response to chemoradiation was evaluated based on RECIST after the end of chemoradiation by chest computerized tomography (CT).
The decision to undergo PCI was individualized. PCI was recommended to patients without progressive disease on a chest CT scan after chemoradiation; however, treating clinicians could decide to omit PCI considering patient performance, disease status, and patient reluctance. The standard dose-fractionation scheme for PCI was 25 Gy in 10 fractions. PCI was planned and delivered with parallel opposed fields. The hippocampus was not spared.
A follow-up visit with chest CT was conducted every 3 months for the first 2 years after the treatment, followed by every 6 months for 3 years. The patients did not undergo brain MRI or PET unless recurrent disease or neurologic symptoms were suspected.
3. Endpoints and statistics
The clinical endpoints analyzed in this study were intracranial recurrence (ICR), ECR, progression-free rate (PFR), and OS. The event for ICR and ECR were defined as the occurrence of the corresponding recurrence. The event for PFR and OS were defined as the occurrence of any disease progression and death from any cause, respectively. Time interval for these clinical endpoints were measured from the date of diagnosis to the date of the event or censoring. The actuarial rates were calculated using the Kaplan-Meier method. Univariate and multivariate analyses of four clinical endpoints were performed to identify potential variables affecting the outcomes using the Cox proportional hazards model. Statistically significant (p < 0.05) and marginally significant (p < 0.1) variables in univariate analysis were included in multivariate analysis. The application of PCI was also included in multivariate analyses for ICR, PFR, and OS as it was a primary variable of interest in this study and expected to affect these outcomes.
PFR event was subcategorized into two types: the first recurrence with ICR (with or without ECR, abbreviated to ICRfirst) and with ECR (without ICR, abbreviated to ECRfirst). ICR confirmed within a month from the confirmation of ECR was considered as simultaneous ICR, and the event was regarded as ICRfirst. The risk of ICRfirst was modeled using a competing risk regression model, setting ECRfirst as a competing event. The association of variables, including PCI and the risk of ICRfirst, was evaluated.
Statistically significant variables in multivariate analysis of ECR were considered as risk factors of ECR. Patients were categorized according to the number of risk factors of ECR. The association of PCI with ICR, OS, and the competing risk of ICRfirst was evaluated according to the number of risk factors of ECR. The statistical significance of the difference in continuous variables was evaluated by t test, and categorical variables were analyzed by chi-square test. Statistical significance was defined as a p value lower than 0.05. All statistical analyses were performed using R ver. 4.2.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Patient characteristics
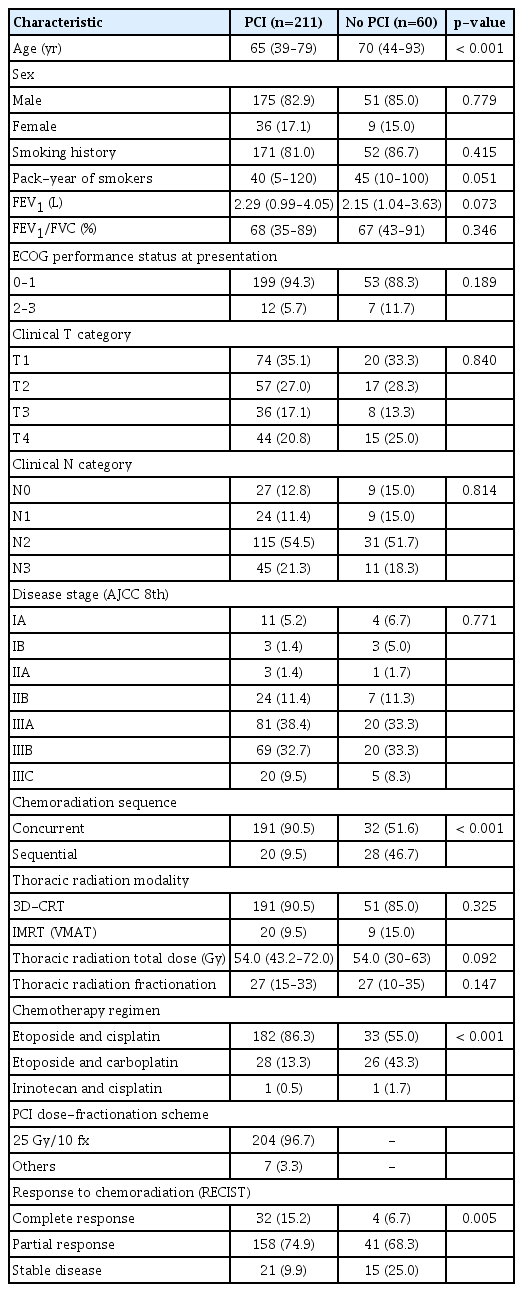
The median follow-up period was 30.7 months (range, 4.9 to 156.7 months). A total of 211 patients (77.9%) underwent PCI, whereas 60 (22.1%) patients did not. The characteristics of the patients according to the application of PCI are summarized in Table 1. The patients were predominantly male (83.4%). Around 82.3% of patients reported a smoking history. Most patients (93.0%) had a good performance status at the initial presentation. A high rate (79.3%) of stage III disease was reported. Some differences in baseline characteristics according to the application of PCI were observed; the age at diagnosis (median, 65 vs. 70 years; p < 0.001), rate of sequential chemoradiation (9.5% vs. 46.7%, p < 0.001), and rate of stable disease after chemoradiation (9.9% vs. 25.0%, p=0.005) were higher among patients without PCI. In the PCI group, 96.7% of patients underwent PCI with a dose-fractionation scheme of 25 Gy in 10 fractions. A total of four patients (1.9%) underwent PCI with 30 Gy in 10 fractions. In addition, two patients (0.9%) received 30 Gy in 12 fractions, and one patient (0.5%) received 20 Gy in 10 fractions.
2. Clinical outcomes
The Kaplan-Meier curves of ICR, ECR, PFR, and OS are illustrated in Fig 1. The actuarial 2-, 3-, and 5-year rates of ICR were 14.5%, 23.2%, and 28.1% with PCI, respectively, and 27.1%, 27.1%, and 32.7% without PCI, respectively. The actuarial 2-, 3-, and 5-year rates of ECR were 48.7%, 53.3%, and 60.8% with PCI, respectively, and 54.9%, 59.6%, and 66.5% without PCI, respectively. No statistically significant difference in ICR (p=0.111) or ECR (p=0.193) according to the application of PCI was observed. The actuarial PFR at 2, 3, and 5 years were 45.4%, 39.3%, and 33.4% with PCI, respectively, and 30.8%, 26.7%, and 21.0% without PCI, respectively. The actuarial rates of OS at 2, 3, and 5 years were 67.8%, 50.0%, and 36.9% with PCI, respectively, and 57.7%, 41.1%, and 32.5% without PCI, respectively. PFR (p=0.008) and OS (p=0.045) were higher with PCI than without PCI.
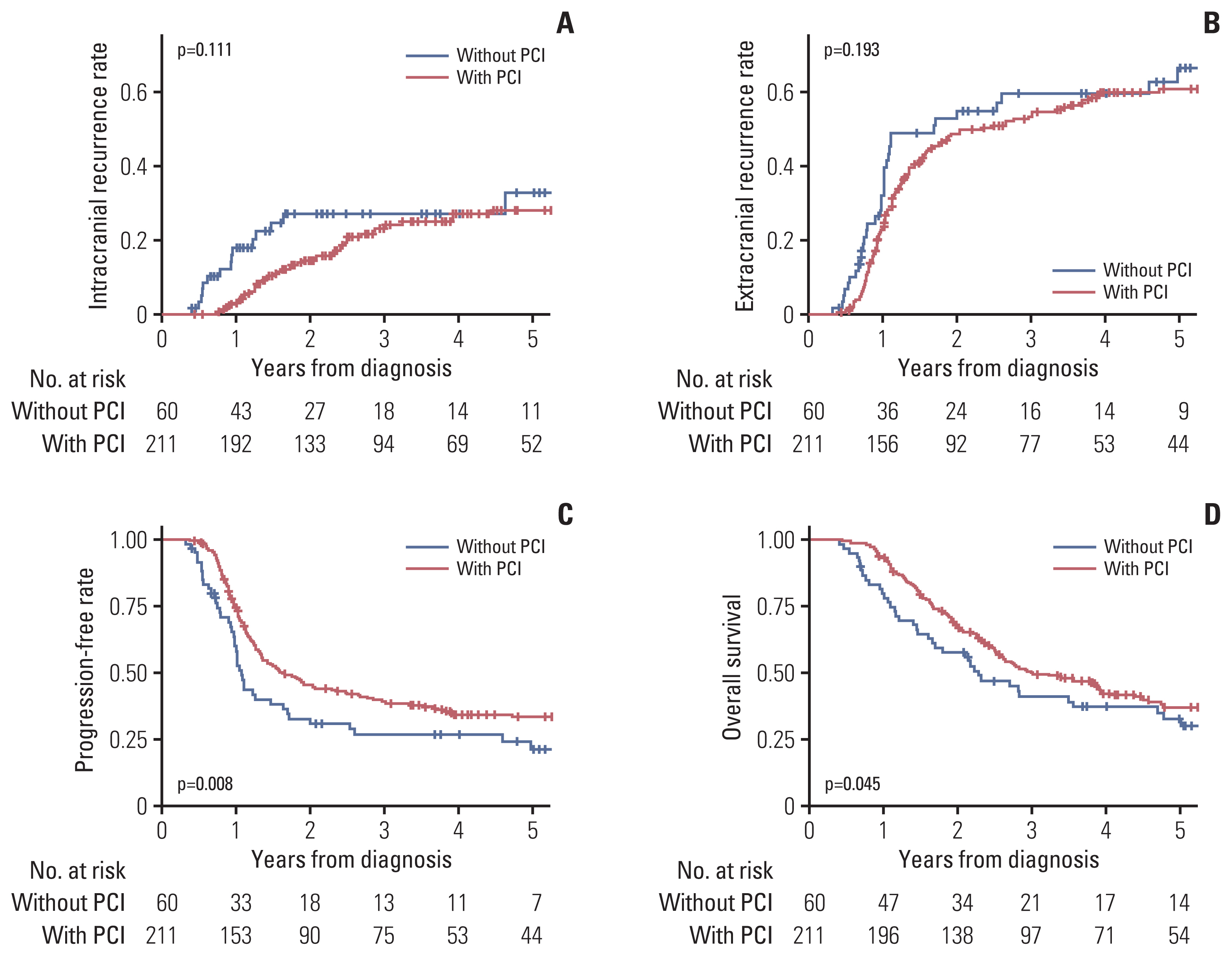
Kaplan-Meier curves of intracranial recurrence (A), extracranial recurrence (B), progression-free rate (C), and overall survival (D) according to the application of prophylactic cranial irradiation (PCI).
The results of univariate and multivariate analyses for four clinical endpoints are summarized in Table 2. In multivariate analysis for ICR, patients with stable disease after thoracic chemoradiation had a significantly higher risk of ICR (hazard ratio [HR], 2.164; 95% confidence interval [CI], 1.154 to 4.057; p=0.016). ECR was associated with disease stage (III vs. I–II: HR, 1.763; 95% CI, 1.152 to 2.696; p=0.009), chemoradiation sequence (concurrent vs. sequential: HR, 1.512; 95% CI, 1.009 to 2.267; p=0.045), and treatment response (stable disease vs. complete response or partial response: HR, 2.097; 95% CI, 1.364 to 3.225; p=0.001). Higher disease stage (HR, 1.793; 95% CI, 1.198 to 2.684; p=0.005) and worse treatment response (HR, 1.961; 95% CI, 1.291 to 2.981; p=0.002) were significantly associated with lower PFR. OS was associated with forced expiratory volume in 1 second/forced vital capacity (HR, 1.399; 95% CI, 1.014 to 1.931; p=0.041), disease stage (HR, 1.931; 95% CI, 1.278 to 2.916; p=0.002), and treatment response (HR, 1.887; 95% CI, 1.247 to 2.857; p=0.003). PCI was not significantly associated with any of the four clinical endpoints in multivariate analysis.
3. Competing risk regression
Data on the risk of ICRfirst and ECRfirst in the competing risk regression model are summarized in S1 Table. The application of PCI was associated with a lower risk of ICRfirst (HR, 0.476; 95% CI, 0.243 to 0.931; p=0.030). No other variable was significantly associated with ICRfirst. Worse treatment response was associated with a higher risk of ECRfirst (HR, 1.849; 95% CI, 1.342 to 2.548; p < 0.001). The actuarial rates of ICRfirst and ECRfirst according to the application of PCI are illustrated in Fig. 2.
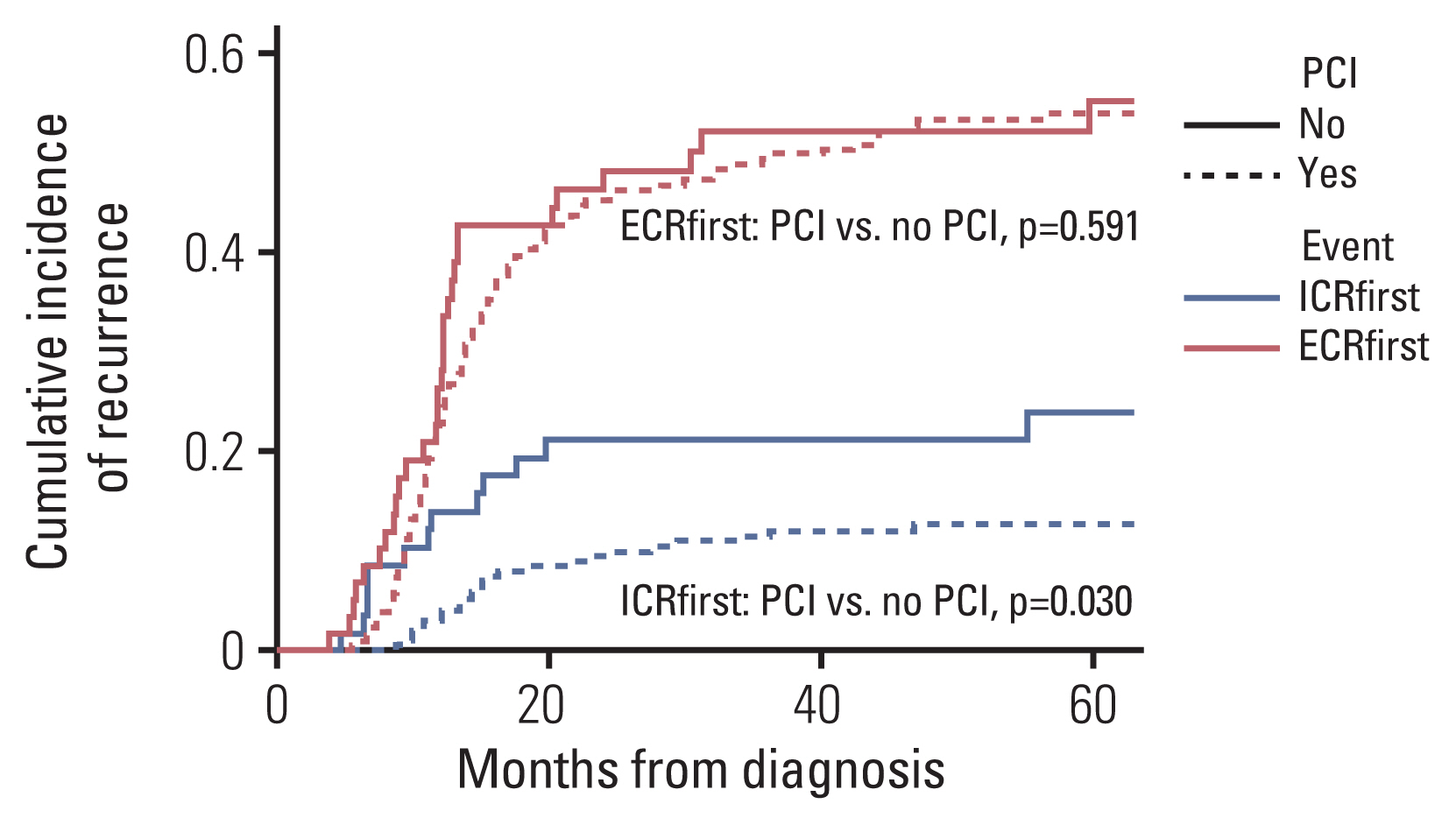
Kaplan-Meier curves of first recurrence with intracranial recurrence (with or without extracranial recurrence, ICRfirst) and first recurrence with extracranial recurrence (without intracranial recurrence, ECRfirst) according to the application of prophylactic cranial irradiation (PCI) based on a competing risk regression model.
4. Stratification according to the risk of ECR
Three variables (disease stage, chemoradiation sequence, and treatment response) significantly associated with ECR in multivariate analysis were defined as risk factors of ECR. The entire cohort was stratified according to the number of risk factors of ECR. Overall, 41 (15.1%), 173 (63.8%), 45 (16.6%), and 12 (4.4%) patients had 0, 1, 2, and 3 risk factors, respectively. The Kaplan-Meier curves of ICR and ECR according to the number of ECR risk factors are illustrated in Fig. 3. Both ICR (p=0.001) and ECR (p < 0.001) were significantly increased with a higher number of ECR risk factors.
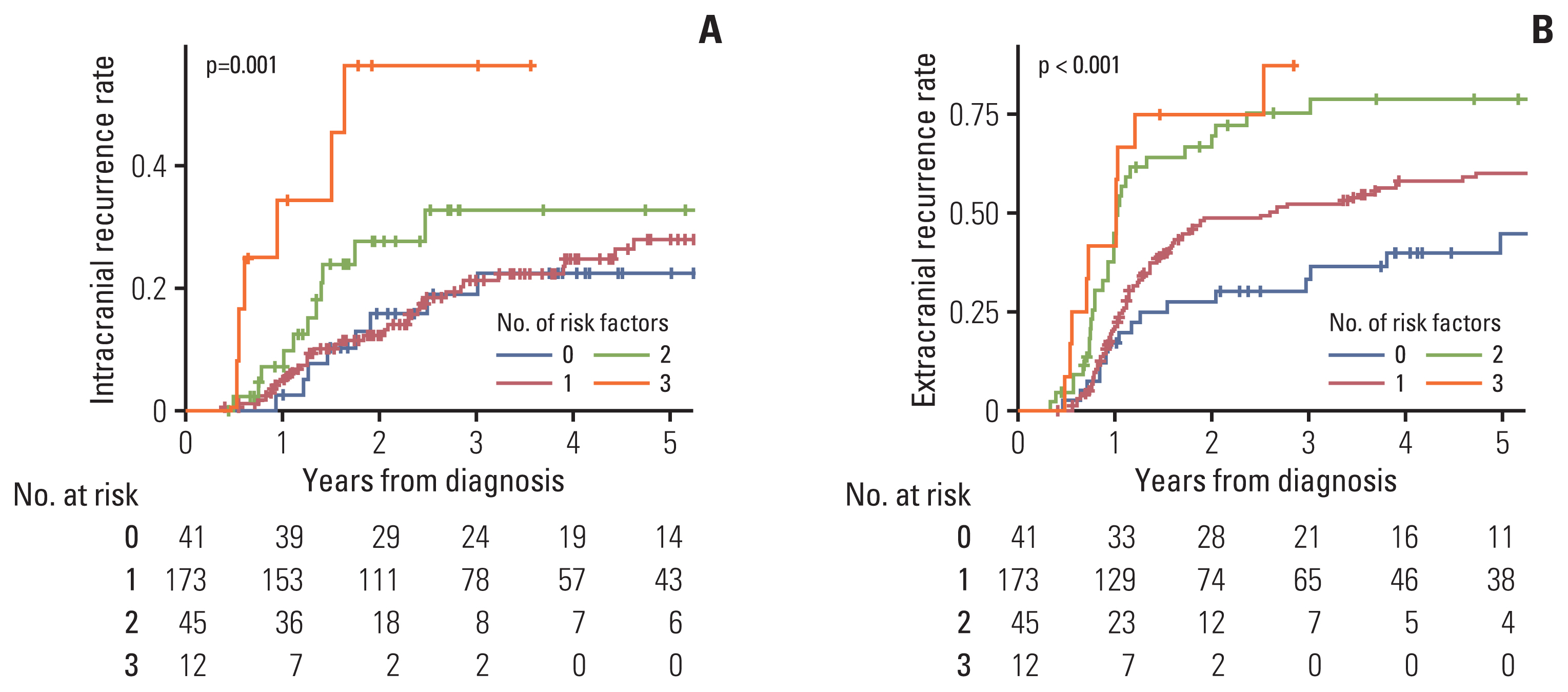
Kaplan-Meier curves of intracranial recurrence (A) and extracranial recurrence (B) according to the number of extracranial recurrence risk factors.
To evaluate the effect of PCI according to the number of ECR risk factors, the ICR, OS, and competing risk of ICRfirst were quantified for each group. For patients without any risk factors of ECR, the application of PCI was associated with marginally higher ICR (p=0.076). Higher OS (p=0.048) and a lower competing risk of ICRfirst (p=0.026) were observed among patients with PCI in this group. The ICR, OS, and competing risk of ICRfirst and ECRfirst of patients without risk factors of ECR are illustrated in Fig. 4. Characteristics of patients without ECR risk factors were summarized in S2 Table. Statistically significant differences in age, clinical T category, thoracic radiation modality, and chemotherapy regimen according to the application of PCI were observed. No statistically significant difference in the ICR, OS, and competing risk of ICRfirst was found for patients with 1, 2, or 3 risk factors. The actuarial rates of these outcomes are illustrated in S3 Fig.

Kaplan-Meier curves of the intracranial recurrence (A), overall survival (B), and competing risk (C) of the first recurrence with intracranial recurrence (with or without extracranial recurrence, ICRfirst) and first recurrence with extracranial recurrence (without intracranial recurrence, ECRfirst) of patients without risk factors of extracranial recurrence according to the application of prophylactic cranial irradiation (PCI).
Discussion
This study evaluated the efficacy of PCI for LS-SCLC patients considering the currently available staging modalities, including brain MRI and PET. In this study, patients who underwent PCI had better PFR and OS; however, there was no difference in ICR. Furthermore, significantly different baseline characteristics were observed between the two groups, and multivariate analysis showed that PCI was not associated with increased PFR and OS, suggesting that the better PFR and OS of patients with PCI may be attributed to different baseline characteristics rather than the actual efficacy of PCI. In the actuarial rates of ICR (Fig. 1A), patients without PCI showed a plateau after an early (< 2 years) increase, and patients with PCI showed a constant increase for 5 years, resulting in a non-significant difference between the two groups. We hypothesized that PCI may not be effective with late brain metastasis after the initial ECR based on this observation. In the competing risk regression model, patients who underwent PCI had a lower risk of ICRfirst, implying that PCI may be effective for decreasing early brain metastasis. We also stratified the cohort according to the risk of ECR. Patients without ECR risk factors also had a low risk of ICR; however, the effectiveness of PCI was maintained in this group. PCI was not effective for patients with ECR risk factors. Therefore, the result suggests that patients with a low risk of ECR may benefit from PCI, which is consistent with the hypothesis. In the hypothesis, the effectiveness of PCI may be associated with the risk of early brain metastasis rather than the absolute risk of brain metastasis. Patients with risk factors of ECR had a relatively higher composition of ECRfirst than ICRfirst in the competing risk regression (S3 Fig.). Although patients with risk factors of ECR had a high risk of ICR, patients with low risk of ECR would benefit from PCI due to a relatively high composition of early brain metastasis.
Several reports have studied the effectiveness of PCI for LS-SCLC with initial staging with brain MRI. Contrary to this study, some of these studies reported a lower risk of ICR with PCI [10–16]. However, some other studies showed no difference in ICR according to the application of PCI, similar to this study. Farris et al. [17] analyzed 92 LS-SCLC patients without progressive disease after definitive treatment and without brain metastasis in both pre- and post-treatment brain MRI. They observed significantly better progression-free survival and better OS with marginal significance among patients who underwent PCI; however, ICR did not show a significant difference. Pezzi et al. [9] evaluated 297 patients with LS-SCLC using propensity score matching and competing risk regression. They reported that there was no difference in OS according to the application of PCI. The exact reason for the varying effects of PCI on ICR in these retrospective studies is unknown. Choi et al. [18] suggested that the effectiveness of PCI for ICR may differ depending on initial staging with PET. They reported that the ICR of patients staged using PET did not decrease following PCI. All patients analyzed in the current study were staged using both brain MRI and PET, and the results are consistent with those of Choi et al. [18]. Nevertheless, several studies that reported the significant effect of PCI on ICR have used PET as a staging procedure [10–13].
According to the actuarial rate of ICR in a study by Farris et al. [17], patients without PCI had a higher rate of ICR initially; however, ICR was subsequently observed among patients with PCI, resulting in a non-significant difference in ICR between the two groups. This pattern was also observed in the current study. We believe that the effect of PCI may be different between early and late brain metastasis and thus performed competing risk analysis. The study by Pezzi et al. [9] also used competing risk analysis to assess the effectiveness of PCI for LS-SCLC. They reported that PCI did not affect ICR risk in competing risk regression modeling. The main difference in competing risk regression between their study and the current study is the competing event; they set death as the competing event, whereas we used ECRfirst to verify the effect of PCI on early brain metastasis. We believe that our approach to differentiating early and late brain metastasis is feasible and warrants further investigation.
According to the results of this study, a subset of early-stage (stage I–II) LS-SCLC patients benefited the most from PCI. Although not confirmative, current clinical guidelines have indicated that the benefit of PCI for early-stage LS-SCLC is not well-established [7,8]. Several retrospective series showed a low risk of ICR in early-stage LS-SCLC. Although some studies compared patients with and without PCI [12,19], others only reported a lower risk of ICR with early-stage disease without evaluating the effectiveness of PCI [20,21]. This study showed that patients with numerically high rate of ICR do not necessarily benefit from PCI and vice versa. Any improvement in clinical outcomes should be evaluated before concluding the effectiveness of PCI, and additional studies are still needed in this context. Some surgical series also stated that very early-stage (stage I or pN0) LS-SCLC may not require PCI [22,23]. In the current study, only patients who underwent chemoradiation were included. Slightly less than 10% of the included patients had stage I disease; however, the disease stage of these patients was not pathologically confirmed. Although pathologically confirmed very early-stage disease may be exempt from PCI due to the low risk of ICR, this does not inevitably extend to clinically staged early-stage LS-SCLC patients who underwent chemoradiation.
There were several limitations in this study. As a retrospective study, the baseline characteristics of patients were not well-balanced. Although we tried to mitigate these differences by multivariate and subgroup analyses, there still may be uncorrected bias. Additionally, the rate of ICR may have been underestimated. ICR evaluation by brain imaging was only performed when neurologic symptoms were present, and there could be asymptomatic ICR events overlooked by clinicians. Nevertheless, we believe that this study provided valuable insights into the management of ICR for LS-SCLC patients.
In conclusion, the application of PCI decreased ICRfirst. PCI may prevent early brain metastasis but not late brain metastasis. In subgroup analysis, patients without any risk factors of ECR (stage III, sequential chemoradiation, and stable disease after thoracic RT) had a low risk of ICR. PCI decreased ICRfirst and increased OS in this patient group alone. Therefore, PCI may be beneficial for patients with a low risk of ECR.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB no. H-1906-154-1044) and Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 10-2021-86) before collecting patient information. Informed consent was waived due to the retrospective nature of this study.
Author Contributions
Conceived and designed the analysis: Lee TH, Kim BH, Kim HJ.
Collected the data: Chung JH, Kim BH, Kim HJ.
Contributed data or analysis tools: Wu HG, Kim S, Lee JH, Keam B, Kim JS, Kim KH, Kim BH, Kim HJ.
Performed the analysis: Lee TH, Chung JH, Kim BH, Kim HJ.
Wrote the paper: Lee TH, Chung JH, Kim BH, Kim HJ.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.