Frequency of Mismatch Repair Deficiency/High Microsatellite Instability and Its Role as a Predictive Biomarker of Response to Immune Checkpoint Inhibitors in Gynecologic Cancers
Article information
Abstract
Purpose
This study was to investigate the frequency of mismatch repair deficiency/high microsatellite instability (MMRd/MSI-H) in gynecologic malignancies and the efficacy of immune checkpoint inhibitors (ICIs) in patients with recurrent gynecologic cancers according to MMR/MSI status.
Materials and Methods
We conducted a multi-center retrospective review on the patients who were diagnosed with gynecologic cancers between 2015 and 2020. Their clinicopathologic information, results of immunohistochemistry staining for MLH1/MSH2/MSH6/PMS2 and MSI analysis, tumor response to treatment with ICIs were investigated.
Results
Among 1,093 patients included in the analysis, MMRd/MSI-H was most frequent in endometrial/uterine cancers (34.8%, 164/471), followed by ovarian, tubal, and peritoneal cancers (12.8%, 54/422) and cervical cancer (11.3%, 21/186). When assessed by histology without regard for cancer types, the frequency of MMRd/MSI-H was 11.0% (38/345) in high-grade serous adenocarcinoma, 38.6% (117/303) in endometrioid adenocarcinoma, and 30.2% (16/53) in carcinosarcoma. A total of 114 patients were treated with ICIs at least once. The objective response rate (ORR) was 21.6% (8/37) in cervical cancer, 4.7% (2/43) in ovarian cancer, and 25.8% (8/31) in endometrial/uterine cancers. Univariate regression analysis identified MMRd/MSI-H as the only significant factor associated with the ORR (28.9% [11/38] vs. 11.8% [9/76]; odds ratio, 3.033; 95% confidence interval, 1.129 to 8.144; p=0.028).
Conclusion
The frequency of MMRd/MSI-H is moderate to high in gynecologic cancers in the Korean population. MMRd/MSI-H could be effective predictive biomarkers in gynecologic cancers of any type.
Introduction
The introduction of immune checkpoint inhibitors (ICIs) has led to a revolutionary change in the oncology field far beyond their remarkable clinical efficacy. In recent years, various ICIs have resulted in an improvement in the overall survival (OS) of patients with a broad range of advanced cancers [1,2]. However, for most types of cancer, only a minority of patients experience a durable response from such treatments while most patients do not benefit significantly. Therefore, attention has been paid to the identification and development of predictive biomarkers of response to ICIs, and more in-depth and comprehensive studies have been conducted in recent years [3,4]. Among the most widely investigated predictive biomarkers of response to ICIs, microsatellite instability (MSI) and defective mismatch repair (MMRd), universal screening tools for identifying Lynch syndrome [5], have been shown to be significant biomarkers for a favorable response to ICIs [6–8].
Mismatch repair deficient tumors have a unique genetic signature, harboring hundreds to thousands of somatic mutations that encode potential neoantigens. These susceptible mutations in repetitive DNA sequences, termed microsatellites, result in high levels of microsatellite instability (MSI-H) [9]. This signature results from primary bi-allelic defects in genes that govern DNA mismatch repair. These tumors arise in individuals with hereditary genetic syndromes, the so-called Lynch syndrome, or more often as sporadic diseases. Tumors with MMRd represent approximately 4% of all diagnosed cancers [10,11]. These tumors vary in frequency across different cancer types. Also, in patients with gynecologic cancers, they occur at a rate of 17%–31% in endometrial cancer, 1%–3% in ovarian cancer, and 2%–4% in cervical cancer [10,11].
The phase II KEYNOTE-158 study of pembrolizumab, an anti–programmed death-1 (PD-1) monoclonal antibody and an ICI, in patients with previously treated, advanced non-colorectal MSI-H/MMRd cancers reported an objective response rate (ORR) of 34.3% (80/233) [7]. In a meta-analysis of 14 studies comprising 939 patients with pre-treated MSI-H tumor, ICIs showed high efficacy that was independent of the tumor type and specific ICI type used, showing a pooled ORR of 41.5% [12]. Pembrolizumab was approved by the United States Food and Drug Administration in May 2017 for the treatment of patients with any type of MSI-H/MMRd solid tumors that have progressed following prior treatment. This marked the first approval of a tumor-agnostic cancer therapy in which treatment is based on a common tumor biomarker rather than the anatomic site of origin. Therefore, it became clear that accurate identification of patients with MMRd/MSI-H tumors is essential for not only screening the genetic background of patients, but also making appropriate therapeutic decisions during disease recurrence.
The frequencies of MMRd/MSI-H in pan-cancer have been reported in several studies [10] and there have been some reports of their frequency in gynecologic cancer patients. However, real-world data comparing the ORR according to MMR/MSI status have not yet been reported in gynecologic cancers. In the present study, we retrospectively assessed the frequency of MMRd/MSI-H in Korean gynecologic cancer patients, and investigated the effect of ICI therapy in recurrent gynecologic cancer with MMRd/MSI-H.
Materials and Methods
1. Study design and patients
We conducted a multi-center, retrospective study at three tertiary academic medical institutions in South Korea. We reviewed the medical records of patients who were diagnosed with gynecologic cancers between January 2015 and December 2020. The collected data included the patient demographics and clinical data on pathologic results, including the results of immunohistochemistry (IHC) staining for MLH1/MSH2/MSH6/PMS2, and MSI analysis. A total of 1,093 patients were included in investigating the frequency of MMRd/MSI-H in gynecologic cancers. Among these patients, we further reviewed the clinicopathologic and radiologic records of those diagnosed with recurrent or persistent gynecologic cancer who underwent treatment with ICIs for at least one cycle. Patients who were treated with ICIs underwent intravenous administration of 200 mg of pembrolizumab every 3 weeks or 3 mg/kg of nivolumab every 2 weeks until disease progression, unacceptable toxicity, or patient withdrawal. The study protocol was approved by the institutional review board of each participating institution (CHA IRB 2020-12-034).
2. Tumor testing
The tumor MMR status was determined by examining the loss of protein expression via IHC staining of four MMR enzymes. Tumors with loss of MMR expression in at least one of those four markers were defined as MMRd. MSI status was determined by the polymerase chain reaction (PCR)–based MSI analysis of DNA from normal and tumor tissues. The analysis was performed using five mononucleotide loci (BAT25, BAT26, NR21, NR24, and Mono27) or five mixed mononucleotide and dinucleotide loci (BAT25, BAT26, D17-S250, D2S123, and D5S346) according to the institution’s established method. Specimens were classified as MSI-H if at least two allelic loci sizes shifted among the five microsatellite markers analyzed. Tumors were classified as MMRd/MSI-H if either MMRd and/or MSI-H were seen. Tumor programmed death-ligand 1 (PD-L1) expression was analyzed using the PD-L1 IHC 22C3 antibody (Agilent Technologies, Inc., Santa Clara, CA) to determine the tumor proportion score (TPS), defined as the percentage of viable tumor cells, or using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Inc., Carpinteria, CA) to determine the combined positive score (CPS), defined as the ratio of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) to the total number of viable tumor cells multiplied by 100. PD-L1 positivity was defined as a TPS ≥ 1% or a CPS > 1.
3. Assessments of response and safety
Baseline tumor assessment was performed before the start of treatment, and response was evaluated via abdominopelvic and/or chest computed tomography scans performed at least every 3 months. Additional imaging studies were performed at the clinician’s discretion if a patient’s clinical symptoms deteriorated. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 by a gynecologic oncologist at each institution. Safety was assessed by retrospectively reviewing charts of laboratory test results and physical examination to detect any possible adverse events (AEs), which were evaluated according to the Common Terminology Criteria for AEs, ver. 4.03.
4. Outcomes
The primary endpoints were the frequency of MMRd/MSI-H tumors in gynecologic cancers and the ORR, defined as the proportion of patients with complete response (CR) or partial response (PR), as assessed using RECIST ver. 1.1. The secondary endpoints included the duration of response, defined as the time from the response to tumor progression or death, whichever occurred first; progression-free survival (PFS), defined as the time from the start of treatment to tumor progression or death, whichever occurred first; and the OS, defined as the time from the start of treatment to death from any cause.
5. Statistical analysis
Efficacy and safety profile analyses included all patients who underwent at least one cycle of treatment. The data were summarized using descriptive statistics or contingency tables for demographic and baseline characteristics, response measurements, and safety. Patients without response data were considered to be non-responders. The duration of response, PFS, and OS were estimated using the Kaplan-Meier method. Univariate logistic regression analyses were performed to identify factors affecting the ORR. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL). Analysis items with p-values less than 0.05 were considered statistically significant.
Results
1. Frequency of MMRd/MSI-H
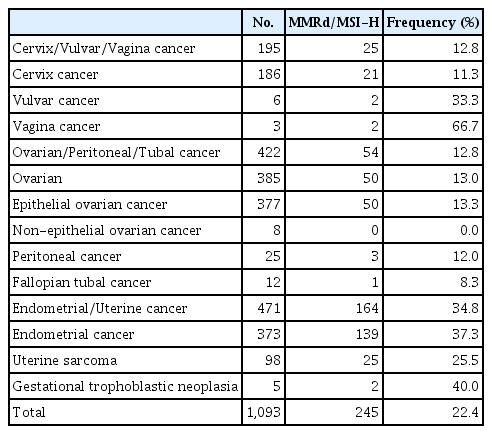
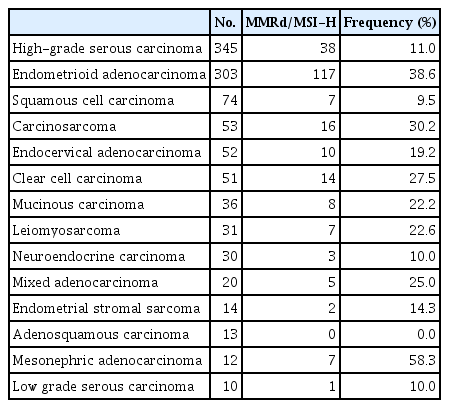
A total of 1,093 patients were included in the analysis. According to the origin of cancer, the frequencies of MMRd/MSI-H were 11.3% in cervical cancer (21/186), 12.8% in ovarian, tubal and peritoneal cancers (54/422), and 37.3% in endometrial cancer (139/373) (Table 1). When assessed by the types of histology regardless of the anatomical cancer origin, the frequency was the highest in mesonephric adenocarcinoma (58.3%, 7/12), 38.6% in endometrioid adenocarcinoma (117/303), 30.2% in carcinosarcoma (16/53), and 27.5% in clear cell carcinoma (14/51) (Table 2). The frequencies of MMRd/MSI-H were 22.1% (216/976) in tumors with non-sarcoma histology, 24.1% (27/112) in tumors with sarcoma histology, and 40% (2/5) in gestational trophoblastic neoplasia (GTN) (S1 Table).
2. Clinicopathologic characteristics
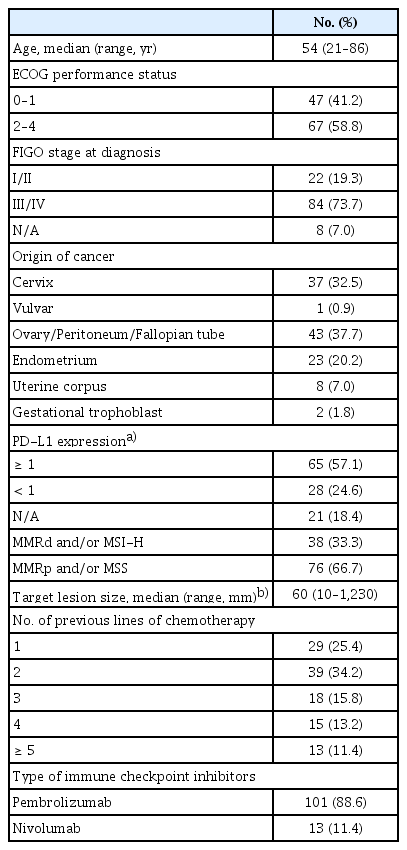
A total of 114 out of 1,093 patients were treated with ICIs for recurrence at least once. The clinicopathologic characteristics of these patients are listed in Table 3. The median age was 54 years (range, 21 to 86 years). Among them, 41.2% (47/114) had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤ 1, and 73.7% (84/114) had stage III or IV disease at the initial diagnosis. In total, eight tumor types were represented among the patients, most commonly ovarian, cervical, endometrial, and uterine corpus (mainly uterine sarcoma) cancers. PD-L1 expression was assessed in 93 patients (81.6%), 65 (69.9%) of whom were PD-L1 positive. Thirty-eight patients (33.3%) had MMRd/MSI-H tumors. The remaining 76 patients were identified as MMR proficient (MMRp)/microsatellite stable (MSS), but received ICI either because their tumor profiles showed PD-L1 positivity or their tumor histology types corresponded to those that have demonstrated response to ICI. The median sum of the target lesions size was 60 mm (range, 10 to 1,230 mm). The median number of lines of prior chemotherapy, including neoadjuvant chemotherapy, was two (range, 1 to 7). The specific agents of ICIs administered were pembrolizumab (88.6%, 101/114) and nivolumab (11.4%, 13/114). As of February 28, 2021, at the time of data cutoff, the median follow-up time was 4.9 months (range, 0.1 to 36.8 months). Eighty-five patients (74.6%) had discontinued ICIs, most commonly due to disease progression. The patients underwent a median of 4 cycles (range, 1 to 40 cycles) of chemotherapy with ICIs.
3. Antitumor activity
In the total population (n=114), five patients (4.4%) achieved CR and 15 (13.2%) achieved PR, resulting in an ORR of 17.5% (Table 4). Among the patients who achieved an objective response, the median time to response was 2.4 months (range, 0.8 to 17.3 months) and the median duration of response was not reached (range, 2.2 to 33.0 months). Among patients with MMRd/MSI-H tumors (n=38), the ORR was 28.9% (3 CRs and 8 PRs). Among patients with MMRp/MSS tumors (n=76), the ORR was 11.8% (2 CRs and 7 PRs) (Table 4).
The response to treatment with ICIs was assessed by anatomical cancer origins and the results are summarized in S2 Table. The ORR was 4.7% (2/43) in ovarian cancer, 21.6% (8/37) in cervical cancer, 26.1% (6/23) in endometrial cancer, 25.0% (2/8) in uterine corpus cancer, and 100.0% (2/2) in GTN. Among patients with MMRd/MSI-H tumors (n=38), the ORR was 33.3% for endometrial cancer (5/15), 33.3% (2/6) for uterine corpus cancer, 14.3% (1/7) for ovarian cancer, and 12.5% (1/8) for cervical cancer (S3 Table).
At the time of data cutoff, 86 (75.4%) patients in the total population had experienced disease progression or death. The median PFS was 2.8 months (95% confidence interval [CI], 2.4 to 3.2), and the estimated PFS rates at 6 and 12 months were 30.1% and 21.4%, respectively (Fig. 1A). Thirty-three patients (28.9%) in the total population had died. The median OS was 35.9 months (95% CI, 16.1 to 55.7) in the total population (Fig. 1B). The OS rates at 6 and 12 months were 77.1% and 61.1%, respectively.
4. Prognostic factors
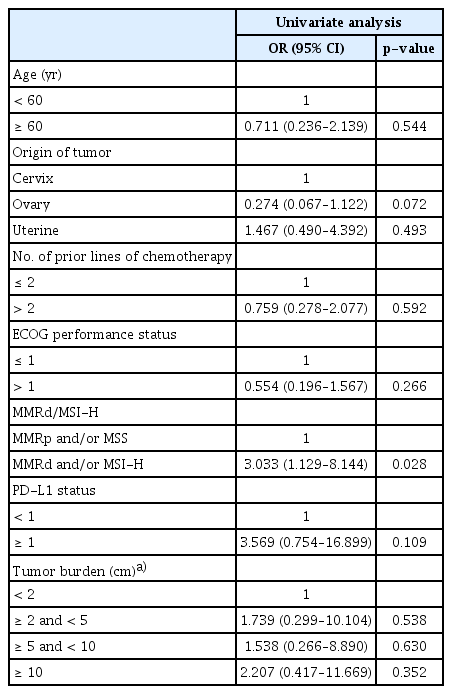
We compared the ORR according to different clinical parameters, including age, tumor origin, the number of previous lines of chemotherapy, ECOG status, PD-L1 positivity, MMRd/MSI-H status, and tumor size (Table 5). MMRd/MSI-H status was the only significant factor found in the univariate regression analyses (odds ratio, 3.033; 95% CI, 1.129 to 8.144; p=0.028).
5. Safety
Treatment-related AEs of any grade and treatment-related AEs of grade ≥ 3 were reported in 42.1% and 4.4% of patients, respectively (S4 Table). There were no treatment-related deaths. The most common AEs of any grade were hypothyroidism (10.5%), anemia (8.8%), fatigue (7.0%), and skin rash (3.5%). The AEs of grades 3/4 were hypothyroidism, anemia, renal insufficiency, colitis, and thrombocytopenia.
Discussion
In two previous studies that evaluated MSI with next-generation sequencing-based methods using data from the Cancer Genome Atlas [10], the frequency of MSI-H was reported to be 3.5%–3.8% for all carcinomas. The frequencies of MSI-H ranged from 28.3% (75/265) to 31.4% (170/542) in endometrial cancer, 1.4% (6/437) to 3.2% (14/436) in ovarian cancer, and 2.3% (7/305) to 2.6% (8/305) in cervical cancer. In the present study, the frequencies of MMRd/MSI-H were 37.3% (139/373) in endometrial cancer, 13.3% (50/377) in epithelial ovarian cancer, and 11.3% (21/186) in cervical cancer (Table 1). The frequency of MMRd and/or MSI-H in endometrial cancer was comparable to those reported in previous studies, and the frequency of ovarian and cervical cancers was higher than that previously reported. However, a previous study which used the classical PCR-based MSI method, which is the same method used in the present study, reported MSI-H rates of 10% for ovarian cancer [13] and 8% for cervical cancer [14]. Therefore, it is possible that these results could be influenced by the difference in MSI analysis methods and reporting methods. Overall, it is noteworthy that endometrial cancer has the highest MMRd/MSI-H frequency.
Mesonephric adenocarcinoma is a rare malignant tumor of the female genital tract, which originates from Wolffian duct remnants. It has been reported to carry a worse prognosis even in the early stages [15,16]. Although the MMRd/MSI-H frequency in mesonephric adenocarcinoma in the present study was 58.3% (7/12), previous studies reported that the frequency of MMRd or MSI-H in mesonephric adenocarcinoma was low [15,16]. This discordance might arise from the small number of cases and the absence of a central pathology review in the present study. Although data are lacking on the response rates of mesonephric adenocarcinoma to ICIs, treatment with ICIs in MMRd/MSI-H mesonephric adenocarcinoma can be considered.
Previous studies reported that the frequency of MMRd/MSI-H in uterine carcinosarcoma was as low as 3.5% (2/57) [11]. However, in the present study, the frequencies of MMRd/MSI-H were 30.2% (16/53) in carcinosarcoma and 22.6% (7/31) in leiomyosarcoma, which were relatively higher (Table 2). In the treatment of gynecologic sarcoma which usually carries a poor prognosis and has no effective therapeutic options at recurrence, it would be helpful to assess the MMRd/MSI-H status and consider treatment using ICIs. GTN comprises a unique group of diseases that arise from the malignant transformation of fetal trophoblasts, cells that originate from the placenta. Recent studies found strong expression of PD-L1 in GTN [17,18] and the frequency of MMRd/MSI-H was 40% (2/5) in the present study (Table 1). The therapeutic response to ICIs in treating chemo-resistant GTN was reported to be favorable [19,20], and there are two ongoing clinical trials on the treatment of chemo-resistant GTN with ICIs (NCT03135769 and NCT04303884).
The mismatch repair pathway plays a crucial role in repairing DNA replication errors. Deficiencies in MMR proteins that cause MSI-H lead to the accumulation of mutations and the generation of neoantigens that might stimulate the antitumor immune response [7]. Tumors with MMRd could induce immune evasion by immune checkpoints, allowing them to escape from the tumor-specific T-cell response [21]. Therefore, using a monoclonal antibody to inhibit immune checkpoints might be an effective therapeutic approach to reversing immune suppression and re-activating the immune system in MMRd/MSI-H tumors regardless of cancer type.
In gynecological cancers regardless of MMRd/MSI-H status, the ORR of anti-PD-1 inhibitors was reported to be low (4%–23%). The respective rates were 4%–12% in cervical cancer [22], 8%–15% in ovarian cancer [23,24], and 13%–23% in endometrial cancer [25,26]. A meta-analysis of 14 studies comprising 939 patients with pre-treated MSI-H cancer reported that the pooled ORR of ICIs was 41.5% (95% CI, 34.9 to 48.4), the pooled median PFS was 4.3 months (95% CI, 3.0 to 6.8), and the pooled median OS was 24 months (95% CI, 20.1 to 28.5) [12]. Another previous study reported that the ORR was 34.3% with a median PFS of 4.1 months and a median OS of 23.5 months among 233 patients representing 27 MMRd/MSI-H tumor types [7]. In that study, the ORRs in endometrial cancer and ovarian cancer were 57.1% (28/49) and 33.3% (5/15), respectively.
In the present study, the ORR of the total population (n=114) was 17.5% and that of the MMRd/MSI-H group (n=38), representing five gynecologic cancer types, was 28.9% (Table 4). The ORRs were 33.3% (5/15) for endometrial cancer with MMRd/MSI-H and 14.3% (1/7) for ovarian cancer with MMRd/MSI-H (S3 Table). The median PFS of MMRd/MSI-H group (n=38) was 2.3 months (95% CI, 0.6 to 4.1), and the median OS was not reached (data not provided). Although it is difficult to directly compare the results of this study with those of other prospective studies, we observed a low overall ORR of ICIs for MMRd/MSI-H tumors. This difference could be influenced by the difference between prospective and retrospective study designs, and by the relatively high MMRd/MSI-H rates observed in the present study. Despite this difference, it was possible to confirm the statistical difference in ORR between MMRd/MSI-H and MMRp/MSS patients.
In the present study, we examined the effects of several factors on ORR: age, cancer type, number of prior lines of chemotherapy, ECOG status, MMRd/MSI-H status, PD-L1 positivity, and tumor size. MMRd/MSI-H was shown to be the only significant factor in the univariate analysis (odds ratio, 3.033; 95% CI, 1.129 to 8.144; p=0.028). Other factors showed no statistically significant associations (Table 5). PD-L1 protein expression on tumor or immune cells has also emerged as a potential predictive biomarker for sensitivity to ICIs [27]. In the present study, the association between PD-L1 expression and ORR could not be confirmed (Table 5). A high tumor mutational burden is another emerging agnostic biomarker with a wider range than MMRd/MSI-H in cancers of any type [28]. Further investigations on such potential biomarker and others are warranted to expand the understanding of profound immune response in malignant diseases.
According to a recent meta-analysis [29], AEs of any grade occurred in 65.8% of patients receiving an ICI, and 16.6% of patients experienced AEs of grade ≥ 3. In the present study, AEs of any grade occurred in 42.1% of patients, and 4.4% of patients experienced AEs of grade ≥ 3. The frequency of AEs in this study was relatively low, which is likely due to the limitations of a retrospective study conducted using chart reviews. Minor AEs might not have been recorded.
The limitations of this study mainly stem from its retrospective design. The lack of independent central pathologic review could also be a confounding factor. Accordingly, there may have been differences in the methods of MMRd/MSI testing and the interpretation of the results among the pathologists at each institution. The frequency of MMRd/MSI-H was higher than those reported in previous studies. The absence of a difference in disease control rate between the MMRd/MSI-H and MMRp/MSS groups (Table 4) might be due to the high MMRd/MSI-H frequency in this study. In addition, MMRd and MSI tests were not performed in all patients. Some patients underwent only one of the two tests. Therefore, it is difficult to conclude that the accurate MMRd/MSI-H frequency was reflected in the present study. Also, the response assessment could not be centralized by an independent central radiologic review. We did not assess the immune response based on the immune RECIST or immune-related RECIST. Although none of the 114 patients raised concerns regarding potential pseudoprogression or hyperprogression even when assessed by the RECIST, the implementation of immune-related response criteria might have portrayed different results. There may also have been differences in the interpretation of the results depending on the types of ICI (pembrolizumab or nivolumab) although both agents belong to the same category and act as anti-PD-1 antibodies. The potential discrepancy between the TPS and CPS to predict response to anti–PD-1/PD-L1 therapy is another limitation. Due to practical issues, the institutions in the present study have adopted different scoring methods. The relatively short follow-up period (median, 4.9 months) is another limitation of the study. Unlike prospective studies, in real-world practice, patients with poor general condition (ECOG PS ≥ 2) are treated with ICIs as the last attempt with short life expectancies. In the present study, more than half (58.8%, 67/114) of the patients had an ECOG PS ≥ 2 (Table 3). As non-responders with poor general condition mostly died soon after treatment with ICIs, the study resulted in a short follow-up period.
Nevertheless, to the best of our knowledge, the present retrospective study of a relatively large, mainly Asian cohort, is the first to evaluate MMRd/MSI-H status as a predictive biomarker for ICIs in gynecologic cancers in a real-world setting. Compared to the known very low MMRd/MSI-H frequencies of ovarian and cervical cancer, in the present study, a relatively high frequency of > 10% was observed. This shows that treatment with ICIs is a potential therapeutic alternative in patients with gynecologic cancers with MMRd/MSI-H. Recently, the combination of ICI and multi-kinase inhibitors has received attention in the treatment of MSS/MMRp tumors, which have a much higher proportion compared to MMRd/MSI-H tumors. Combined therapy comprising pembrolizumab and lenvatinib (an oral multi-kinase inhibitor) for MSS/MMRp recurrent endometrial cancer has been found to yield favorable outcomes among 37.2% (35/94) of patients [30]. As such study, new combination therapeutic strategies are also being specified for MSS/MMRp tumors.
The present study has shown that the frequency of MMRd/MSI-H in gynecologic cancers is moderate to high in Korea. MMRd/MSI-H status was confirmed to be a predictive biomarker for ICI therapy in gynecologic cancers. Further studies are warranted to discover other predictive biomarkers for ICI therapy in gynecologic cancer.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the institutional review board (CHA IRB 2020-12-034) and adhered to the principles in the Declaration of Helsinki. A waiver to require informed consent was obtained.
Author Contributions
Conceived and designed the analysis: Choi MC, Lee JW, Lee C.
Collected the data: Noh JJ, Kim MK, Choi MC, Lee JW, Park H, Jung SG, Joo WD, Song SH, Lee C.
Contributed data or analysis tools: Noh JJ, Kim MK, Choi MC, Lee JW.
Performed the analysis: Choi MC, Lee JW, Park H, Jung SG, Joo WD.
Wrote the paper: Noh JJ, Kim MK, Choi MC, Lee JW.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.