Guidelines for Cancer Care during the COVID-19 Pandemic in South Korea
Article information
Abstract
At the end of 2019, the cause of pneumonia outbreaks in Wuhan, China, was identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In February 2020, the World Health Organization named the disease cause by SARS-CoV-2 as coronavirus disease 2019 (COVID-19). In response to the pandemic, the Korean Cancer Association formed the COVID-19 task force to develop practice guidelines. This special article introduces the clinical practice guidelines for cancer patients which will help oncologists best manage cancer patients during the COVID-19 pandemic.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is an ongoing global issue [1,2]. In South Korea, the first COVID-19–positive patient was diagnosed on January 20, 2020 [3]. Since then, COVID-19 has caused serious health concerns in the treatment of cancer patients [4–7]. Quarantine protocols have been issued by the South Korean Government, including the Central Disaster and Safety Countermeasures Headquarters [3].
The guideline presented here was developed to help oncologists manage cancer patients who have a high mortality rate. Other countries have developed COVID-19–specific cancer guidelines [7–9]. The difference in situation and management in South Korea prompted the development of guidelines specifically for South Korean oncologists. We hope that these guidelines will provide clinical guidance to cancer care during the ongoing COVID-19 pandemic.
Method
The guideline was first written in Korean in April 2020 by clinical experts who were members of the Korean Cancer Association and the National Cancer Center and include general guidelines for cancer care and specific recommendations for surgery, chemotherapy, radiotherapy, pediatric oncology, cancer screening, and clinical trials. Many of the information provided here were developed from experts’ opinions and experience during the time when COVID-19 pandemic hit Korea in March. The guideline was then translated to English so that the clinicians around the world may have access to this guideline. All authors unanimously agree to the recommendations as outlined in the guideline. In general, the guideline for screening, treatment and follow-up for cancer care primarily depends on two factors: (1) the shortages in medical devices or healthcare workers, and (2) whether the patient is positive for COVID-19 (Fig. 1).
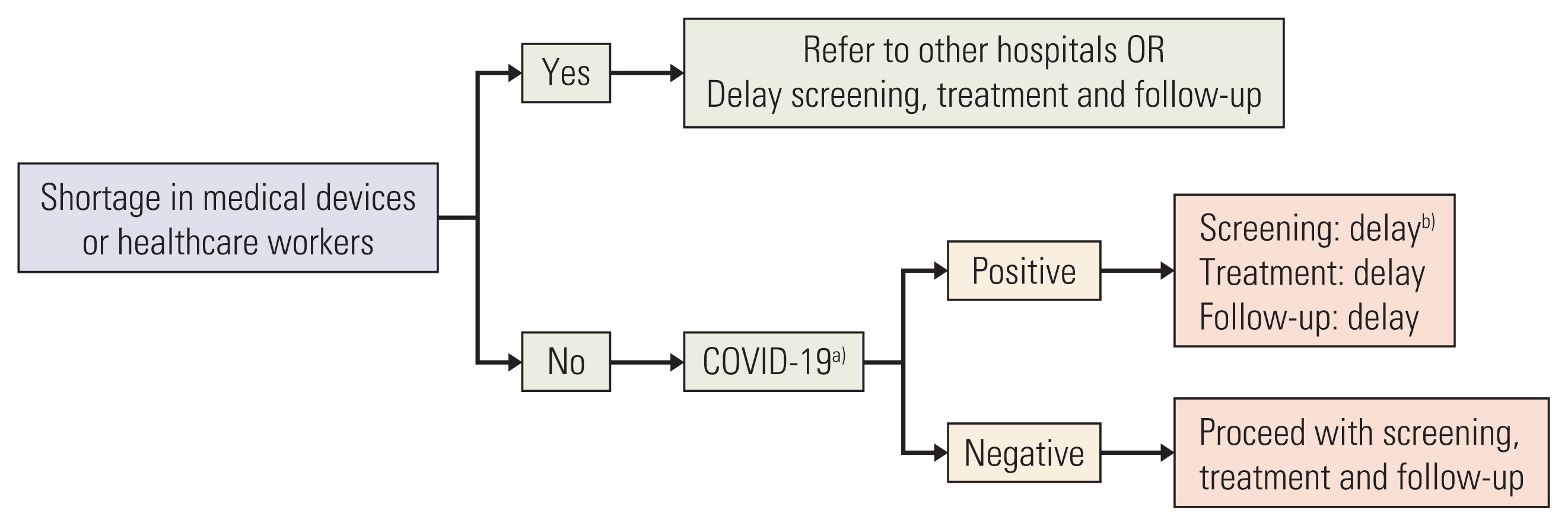
Basic algorithm for screening, treatment and follow-up during coronavirus disease 2019 (COVID-19). a)Indications for COVID-19 screening includes one of the following: (1) symptoms related to COVID-19, (2) history of recent overseas travel within 14 days, contact with a COVID-19 patient, b)Delay: Refer to Table 2 for summary of recommendations for cancer screening.
General Guidelines
1. Prevention
Guidelines for cancer patients may be modified during massive regional or national COVID-19 outbreaks. Clinicians should discuss the benefits and risks of cancer treatment with patients before making treatment decisions. Both healthcare workers and patients should wash their hands and wear masks during face-to-face communication. The clinic and facilities treating patients must be large, open, and well-ventilated. The 2-m social distancing rule should be applied. Patients should be educated about the symptoms of COVID-19.
2. Treatment
COVID-19–positive cancer patients should be treated in the same manner as COVID-19–positive patients without cancer. Stop all cancer treatments such as elective surgery, chemotherapy, and radiotherapy if a cancer patient is diagnosed with COVID-19. Patients should be admitted to hospital since they are at a high risk of severe disease. Cancer treatment may resume once they recover from COVID-19. Patients who have completed their cancer treatment or are under surveillance for recurrence should not postpone routine tests or hospital visits. Postponing follow-up tests and hospital visits should only occur if there are shortages of medical devices or healthcare workers.
3. Facility
Healthcare facilities should establish a diagnostic laboratory in which cancer patients suspected with COVID-19 can be tested rapidly. Establish a network for patient referral and swiftly refer to another hospital when necessary. Prevent delays in patient treatment if there is a COVID-19 outbreak in healthcare facilities. Clinicians are recommended to communicate via telemedicine. Options include telephone-only consultation, virtual check-in, and e-visits.
Separate triage stations for COVID-19 screening should be established for both outpatient clinics and emergency departments. Patients should be screened if they have the following conditions: (1) symptoms related to COVID-19, (2) a history of recent overseas travel within 14 days, or (3) contact with a COVID-19 patient. Once a patient is tested negative for COVID-19 disease, the patient should be referred to the outpatient clinic or admit to the ward, as appropriate.
Surgery
Surgery should not be postponed due to the potential risk of cancer progression. At the discretion of doctors, surgery may be delayed, but the decisions must be based on an individual basis. Emergency operations should never be delayed. Prior to surgery, factors such as the center’s infrastructure to treat COVID-19–positive patients, the status of healthcare workers, and availability of the intensive care units and general wards should be considered. Prior to elective surgery, all patients should be screened for COVID-19.
Clinicians should wear personal protective equipment (PPE) during surgery for febrile patients waiting for COVID-19 test results [10]. The least possible number of staff should be allowed to assist during surgery. The patient should recover in the operation room after surgery. While awaiting the COVID-19 test results, the recovered patient should be send to an isolation ward via a safe route. The patient should be sent to the general ward only after testing negative for COVID-19.
The following are necessary equipment for healthcare workers, including surgeons, anesthesiologists, and nurses: PPE including double gloves, N95 face mask, goggles, surgical cap, surgical gown, and shoe covers. For COVID-19 patients or suspected COVID-19 patients, medical staff should wear level D PPE, powered with an air-purifying respirator (PAPR). Alternatives to surgery, such as cancer drugs and radiation treatment, should be considered in the case of large-scale COVID-19 outbreaks that result in a shortage of healthcare workers.
Chemotherapy
Chemotherapy should be administered on an individual basis since COVID-19 outbreaks and medical resources can vary regionally. During epidemic breakout of COVID-19, patients with a low risk of COVID-19 should continue routine chemotherapy. During pandemic COVID-19 era, where there is a high risk for COVID-19 leading to a shortage of medical resources and healthcare workers, patients are advised to postpones admission to wards. They are encouraged to be diagnosed and treated in outpatient clinics to prevent COVID-19. Outpatient clinics may be rescheduled. Patients should minimize hospital visits by communicating via telemedicine, preferably by phone or virtually, rather than having face-to-face interaction.
Clinicians are advised to implement treatment strategies with fewer hospital visits. Clinicians may change the route of chemotherapy (hormonal treatment) from intravenous to oral and treat in outpatient clinics. If possible, use chemotherapy with longer treatment cycles to avoid frequent hospital visits.
Cancer patients receiving adjuvant chemotherapy should not delay treatment except during a shortage of medical resources, in which case the patients should be referred to other hospitals. The clinicians should discuss the benefits and risks of omitting or delaying treatment, including the possibility of recurrence to the patients.
Patients with advanced or recurrent solid tumors requiring palliative chemotherapy should continue treatment. During a shortage of medical resources, chemotherapy may be postponed only if the primary physician believes that the disease progression is slow and the cancer-related symptoms are minimal. However, palliative chemotherapy should not be delayed if the patient is experiencing symptoms or rapid progression. Implement ways to minimize emergency department visits related to chemotherapeutic side effects.
In patients with febrile neutropenia after chemotherapy, clinicians should exercise caution because it is difficult to distinguish COVID-19 from other viral infections. Clinicians are advised to use granulocyte colony-stimulating factor. Dose reduction or delay future chemotherapy should be considered in the next treatment cycle. COVID-19 must be screened for asymptomatic patients who need admission to ward. However, it is not mandatory for asymptomatic patients receiving chemotherapy in the outpatient clinic.
Radiotherapy
Generally, patients with de novo tumors requiring radiotherapy should not mandatorily delay radiation treatment. However, radiotherapy may be withheld and substituted with other treatment if the patient is asymptomatic or in poor general condition. During a shortage of medical resources, radiation may be delayed at the discretion of the radiation oncologist. With the exception of emergency and life-threatening conditions such as spinal cord compression, cauda equina syndrome, increased intracranial pressure, superior vena cava syndrome, obstruction of airways, hemoptysis, and tumor bleeding, all other elective radiotherapy may be delayed. For hypofractionated radiotherapy, the dosage of radiation maybe increased and the frequency of treatment decreased.
For patients who are tested positive for COVID-19, radiation treatment may be withheld unless they require the immediate interventions due to emergency and life-threating conditions. Treatment may be resumed once the patient recovers from COVID-19. Radiologists may consider increasing the total dose of radiation after the unexpected “treatment holiday.”
After completion of radiotherapy, follow-up visits may be delayed or minimized at the discretion of radiation oncologists. In patients with stable condition who do not have any side effects from radiation treatment, less stringent follow-up visits are recommended with a follow-up of 3-month period.
Pediatric Oncology
Currently, there are no guidelines for treatment of pediatric COVID-19 patients. Generally, pediatric cancer patients higher risk for COVID-19 complications than healthy adults [11]. In infants, children, and adolescents, the symptoms of COVID-19 appear as respiratory symptoms, such as fever, cough, and dyspnea, and non-respiratory symptoms, such as vomiting and diarrhea.
Chemotherapy in pediatric oncology patients should not be delayed. Patients who received chemotherapy or surgery within a month of COVID-19 are likely to develop more severe infection. Therefore, patients with symptoms such as fever and cough should be treated promptly. Minimize hospital visits by delaying outpatient clinic appointments or substituting with telephone-only visits for patients who have completed chemotherapy. Maintain regular follow-up visits. Consider switching from intravenous drugs to oral drugs, if possible.
Although there are no standardized guidelines for pediatric patients preparing for stem cell transplantation, the European Society for Blood and Marrow Transplantation guidelines recommend that both donor and recipient are screened for COVID-19 [12]. Prior to stem cell transplantation, optimize blood storage to prevent a lack of blood supply during transfusions.
All treatments, including non-elective surgery, chemotherapy, and radiotherapy should be withheld in COVID-19–positive pediatric patients, and COVID-19 should be treated immediately. Admit the patients to hospitals since they are at a high risk of serious disease. Patients should be rigorously monitored even after treatment, since they are immunocompromised and can spread COVID-19 even if they are asymptomatic. Cancer-related treatments may resume once they recover from COVID-19.
Cancer Screening
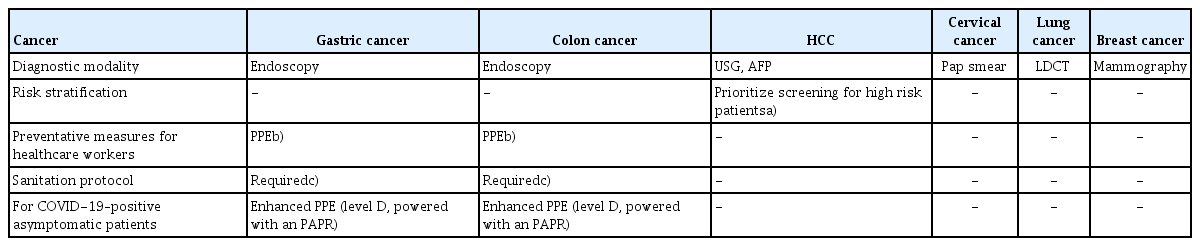
The guidelines for cancer screening are outlined in Table 1. In general, cancer screening should not be delayed in healthy patients. Asymptomatic patients who are unlikely to have COVID-19 may proceed with cancer screening without COVID-19 results. Screening methods such as Pap smear, mammography, and low-dose computed tomography for cervical cancer, breast cancer, and lung cancer respectively, may be postponed if there is a shortage of medical resources. With the exception of screening with endoscopy, all cancer screening should be delayed in COVID-19–positive patients until they are tested negative (Table 2).
For gastric and colon cancer screening, check if the patients have fever or respiratory symptoms and screen for COVID-19. Healthcare workers must use PPE during the procedure and follow sanitation protocols during endoscopic procedures. For COVID-19–positive asymptomatic patients, proceed with the endoscopy in a setting of enhanced PPE (level D, powered with an PAPR). Follow the protocols for sanitation and facility cleaning as recommended in Table 3. For patients screening for hepatocellular carcinoma, risk factors should be stratified. Patients with low risk may postpone screening. During a shortage of medical resources, prioritize screening patients who have elevated α-fetoprotein levels, liver cirrhosis, and chronic hepatitis B. Encourage patients who are current smokers and are at high risk of both lung cancer and COVID-19, to stop smoking.
Clinical Trials
Patient safety is the foremost priority during clinical trials. The principal investigator, sub-investigator, and clinical research coordinator must follow the general guidelines for COVID-19 prevention. The trial sponsors and clinical trial centers, including the Institutional Review Board (IRB) members and the principal investigators, should adhere to the protocol as outlined by the Ministry of Food and Drug. The investigators should consult sponsors about the possibility of protocol deviations during the COVID-19 pandemic. Clinicians should enroll and treat the subject as outlined in the clinical trial protocol. Assess and ensure the safe shipment of investigational products. Ensure the safety of patients and staff by taking precautionary measures during the storage, shipment, and delivery of biological samples. One method is to sanitize the external packages received.
Conclusion
The summary of general guidelines, surgery, chemotherapy, radiotherapy, pediatric oncology, cancer screening, and clinical trial for cancer patients during the COVID-19 pandemic is outlined in Table 4. Lastly, individualized treatment strategies and discretion of clinicians are needed to adjust to the challenges faced with maintaining cancer care in the COVID-19 era.
Notes
Author Contributions
Conceived and designed the analysis: Jung M, Kim JH, Kim BH, Kim Y, Kim YS, Kim BC, Kim J, Moon SH, Park KU, Park M, Park HJ, Sim SH, Yoon HM, Lee SJ, Lee E, Chun JY, Chung YK, Jung SY, Chung J, Lee ES, Chung HC, Yun T, Rha SY.
Collected the data: Kim JH, Kim BH, Kim Y, Kim YS, Kim BC, Kim J, Moon SH, Park KU, Park M, Park HJ, Sim SH, Yoon HM, Lee SJ, Lee E, Chun JY, Chung YK, Jung SY, Chung J, Lee ES, Chung HC, Yun T, Rha SY.
Contributed data or analysis tools: Kim JH, Kim BH, Kim Y, Kim YS, Kim BC, Kim J, Moon SH, Park KU, Park M, Park HJ, Sim SH, Yoon HM, Lee SJ, Lee E, Chun JY, Chung YK, Jung SY, Chung J, Lee ES, Chung HC, Yun T, Rha SY.
Wrote the paper: Lee JB, Jung M, Kim JH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
We would like to thank all the board members from the Korean Cancer Association and the Office of Public Relations and Collaboration at the National Cancer Center, South Korea, for supporting the development of these COVID-19 clinical practice guidelines for cancer care.