AbstractPurposeThis study aimed to evaluate the effects of bladder cuff method on oncological outcomes in patients who underwent radical nephroureterectomy (RNU) for upper tract urothelial carcinoma.
Materials and MethodsThe records of 1,095 patients treated with RNU performed at our hospital between 1994 and 2018 were retrospectively reviewed; 856 patients with no bladder tumor history were enrolled in the present study. The management of bladder cuff was divided into two categories: extravesical ligation (EL) or transvesical resection (TR). Survival was analyzed using the Kaplan-Meier method and Cox regression analyses were performed to determine which factors were associated with intravesical recurrence (IVR)–free survival (IVRFS), cancer-specific survival (CSS), and overall survival (OS).
ResultsThe mean patient age was 64.8 years and the median follow-up was 37.7 months. Among the 865 patients, 477 (55.7%) underwent the TR and 379 (44.3%) the EL. Significantly higher IVRFS (p=0.001) and OS (p=0.013) were observed in the TR group. In multivariable analysis, IVR, CSS, and OS were independently associated with the EL. Among 379 patients treated with the EL, eight underwent remnant ureterectomy. Based on radical cystectomy–free survival, significant difference was not observed between the two groups. However, significantly higher IVRFS was observed in the TR group when the tumor was located in the renal pelvis.
IntroductionUpper urinary tract urothelial carcinoma (UTUC) is a rare disease and accounts for 7%–8% of all renal tumors and 5% of all urothelial malignancies [1–3]. The carcinoma can arise from the urothelial cells of the renal calyces, renal pelvis, or ureter [4]; thus, the recurrence of bladder urothelial carcinoma is common, occurring in 15%–50% of patients after treatment [5,6].
Radical nephroureterectomy (RNU) with bladder cuff excision (BCE) is the gold standard for the treatment of non-metastatic UTUC [7–9]. Several BCE methods have been described [4,10], however, the optimal BCE method is unclear. Complete removal of intramural portion of distal ureter should be implemented although no specific method about BCE is recommended in the current guidelines [5,11]. Several groups have evaluated the effects of different distal ureter approaches on oncological outcomes [1,7,12–19]. To date, the oncological outcomes of these method have been evaluated in only a few studies. Among them, the extravesical approach implies the possibility of incomplete elimination of intramural ureter. In a recent systematic review, extravesical incision of the bladder cuff which involves dissecting the distal ureter and bladder cuff extravesically was still performed in 30%–60% of patients who underwent RNU and intravesical incision of the bladder cuff is associated with improved intravesical recur free survival [20].
In the present study, the effects of different distal ureter approaches on the oncological outcomes was assessed in a large, single center cohort of patients treated with RNU.
Materials and Methods1. PatientsAfter Institutional Review Board approval, the records of 1,095 patients who underwent RNU with BCE for UTUC at our center from 1994 to 2018 were retrospectively reviewed. Patients with prior bladder cancer history, prior cystectomy, or systemic metastasis at presentation were excluded. Patients were also excluded if they received preoperative chemotherapy or radiotherapy or if final pathology did not reveal UTUC. Lymph node status was not considered an exclusion criterion. Patients underwent lymph node (LN) dissection if clinically significant LN is indicated on the preoperative computed tomography (CT). Finally, 856 patients were included in the present study. Descriptions of pathological characteristics (i.e., confirmation of transitional cell carcinoma histology, tumor grade, presence of carcinoma in situ, lymphovascular invasion [LVI], location, unifocality, and multifocality) were based on pathologic reports completed after surgery.
2. Surgical techniqueRNU was performed using BCE. The distal ureter was removed either through an extravesical ligation (EL) of bladder cuff or transvesical resection (TR) of bladder cuff. Pluck Technique which is transurethral resection of ureteral orifice was not used in our center. TR of bladder cuff was defined as visually confirming the ipsilateral ureteral orifice before fully excising the bladder cuff. This procedure included two types of surgical method: (1) creating an anterior cystotomy and then excision of the intramural portion of ureter (two incision on bladder); (2) or creating an cystotomy directly at the upper portion of far distal ureter and the intramural portion of the ureter was completely dissected (one incision on bladder). Bladder was closed with a two-layer suture and we confirm a watertight closure through filling water in bladder.
In the present study, the EL of bladder cuff was classified as extravesical control of the premural ureter with a surgical device. With maximally traction on the ureter close to the bladder, the distal ureter was dissected with clip, Hem-o-lok, or tie. In this method, bladder was not incised and the ureteral orifice could not be visually confirmed.
During nephrectomy, the ureter is identified and clipped/or ligated at the level of the proximal ureter. The choice of bladder cuff method was based on the surgeon’s preference. However, if the tumor involved the ureterovesical junction, all surgeons removed the distal ureter using the TR of bladder cuff. The TR was performed after RNU via a Gibson or Pfannenstiel incision.
3. Follow-upPatients were followed up, generally, every 3–4 months for the first year following RNU, every 6 months from the second to the fifth year, and annually thereafter. Follow-up after RNU consisted of history, physical examination, routine blood test, urine cytology, chest X-rays, cystoscopic evaluation of the bladder, and abdomino-pelvic CT for the evaluation of contralateral upper urinary tract. Adjuvant chemotherapy was considered in patients with higher than pT2 category or with pN+.
4. Oncological outcomesOutcome parameters were assessed for intravesical recurrence (IVR), death due to disease, and death due to other causes. IVR was defined as any urothelial disease identified after RNU in the bladder. Cause of death was determined by physicians who performed the treatment and by chart review corroborated with death certificate. Time for each parameter was measured from the day of RNU to diagnosis of IVR or death.
5. Statistical analysisDifferences in continuous variables across distal ureter approaches were assessed using the Student’s t test. The chi-square test was used to evaluate the association between categorical variables and the two approaches (TR and EL). IVR-free survival (IVRFS), cancer-specific survival (CSS), and overall survival (OS) curves were generated using the Kaplan-Meier method and compared using the log-rank test. The same statistical analysis was used to evaluate radical cystectomy (RC)–free survival and IVRFS based on distal ureter approach used in several preoperative situations. Multivariable Cox regression models were used to measure outcomes after RNU. All reported p-values were two sided and statistical significance was set at 0.05. Statistical analyses were performed using SPSS ver. 21.0 (IBM Corp., Armonk, NY).
Results1. Patient baseline characteristicsPatient baseline characteristics and pathologic findings are shown in Table 1. The mean patient age was 64.8±10.8 years and 27.7% were female. The median follow-up was 37.7 months. Among the 856 patients, 477 (55.7%) underwent the TR and 379 (44.3%) underwent the EL. Groups differed significantly in the proportion of laparoscopic cases, follow-up duration, and time from RNU to IVR (p < 0.05).
2. Oncological outcomesIVRFS for the TR and EL at 5 years were 59.9% and 49.3%, respectively (p=0.008) (Fig. 1A). At 10 years, the IVRFS was 56.3% and 45.5% for the TR and EL, respectively (p=0.028) (Fig. 1A). CSS estimates at 5 years after RNU were 82.0% and 73.8% for the TR and EL, respectively (p=0.019) (Fig. 1B), and at 10 years after RNU were 74.7% and 69.4%, respectively (p=0.213) (Fig. 1B). OS estimates at 5 years after RNU were 79.7% and 68.0% for the TR and EL, respectively (p=0.001) (Fig. 1C), and at 10 years after RNU were 61.2% and 58.4%, respectively (p=0.648) (Fig. 1C).
3. Factors affecting IVR, CSS, and OSIn multivariable Cox regression analysis, the EL was associated with IVR (hazard ratio, [HR], 1.40; 95% confidence interval [CI], 1.12 to 1.75; p=0.003) compared with TR. Other predictors of IVR based on multivariable analysis included age, preoperative ureteroscopy (URS), tumor location, and pN category (Table 2). The EL was also associated with worse CSS and OS (HR, 1.47; 95% CI, 1.06 to 2.05; p=0.022 and HR, 1.52; 95% CI, 1.14 to 2.03; p=0.005, respectively). CSS and OS were also significantly associated with age, surgical approach, tumor location, pT category, pN category, and LVI in multivariable analysis.
4. RC-free survival and the rate of remnant ureterectomyThe number of patients who underwent RC after RNU in the TR and EL procedure groups was 11 (2.3%) and 10 (2.6%), respectively. RC-free survival estimates at 5 years after RNU were 97.3% and 95.7% for the TR and EL, respectively (p=0.352) (Fig. 2), and at 10 years after RNU were 95.0% and 95.7%, respectively (p=0.742) (Fig. 2). In addition, the number of patients who underwent remnant ureterectomy after RNU in the EL group was eight (2.1%). The mean period from RNU to remnant ureterectomy was 32.4 months.
5. IVRFS in specific preoperative situations between the two groupsIn the subgroup analysis according to the location of tumor, the TR group showed significantly improved IVRFS in patients with renal pelvis tumor (p=0.003), but not in the subgroup of ureter tumor (p=0.101). In patients with a single tumor, TR group had less IVR than EL group (p=0.002), but there was no difference in patients with multiple lesions (p=0.227).
DiscussionTotal excision of the distal ureter with its intramural portion, the ipsilateral ureteral orifice, and bladder cuff is considered necessary for optimal management of UTUC [7,8,13]. Currently, several surgical approaches are used for the management of distal ureter during RNU [1,4,5,7,12,16,21]. Among the approaches, including intravesical and extravesical BCE, involve opening the bladder [5,7,21]. However, many surgeons perform RNU and distal ureter approaches without incising the bladder but resecting the distal ureter outside the bladder near the orifice. In addition, the oncological outcomes of this method have been evaluated in only a few studies [5,20]. In the present study, the TR was associated with improved IVRFS and OS compared with EL. In addition, the TR had higher CSS although without statistical significance. The results demonstrated the incomplete distal ureteric resection is closely associated with poor oncological outcomes such as IVR, which is in agreement with previous study results. Reportedly, if the distal ureter is not appropriately removed, recurrence rates can range from 33%–75% in the ureteric remnant even in the absence of positive surgical margins [21–27].
Multivariable analysis showed the bladder cuffing method is significantly associated with IVR, CSS, and OS. In addition, N category, tumor location, and age played a significant role in IVR, CSS, and OS. Although LVI and T category (higher than T2) did not significantly affect IVR, these factors affect CSS or OS. Possibly, either IVR is not significantly associated with LVI and T category higher than T2, or occurrence of IVR is not significantly different between low and high T category. Conversely, preoperative URS significantly affected IVR but not CSS and OS. Similarly, preoperative URS was significantly associated with IVR in our previous study [28], which was confirmed in this present study. Using diagnostic tools such as CT instead of performing preoperative URS could prevent the possibility of bladder tumor recurrence following RNU.
The multivariable analysis demonstrated the type of bladder cuff significantly affects IVR and EL has detrimental effects on recurrence. Therefore, RC is expected to be performed more on patients who receive RNU using the EL due to greater IVR than in patients who receive the TR. However, RC was performed in 11 of 477 patients (2.3%) who received the TR and in 10 of 379 patients (2.6%) who received the EL. Significant difference was not observed in RC performance rate and RC-free survival. Therefore, although there was high recurrence rate of low T category bladder tumor in the EL group, recurrence of higher T category (higher than T2) bladder tumor was not likely to be affected by the type of bladder cuff. However, the results can be interpreted differently. Although the EL causes higher bladder tumor recurrences, transurethral resection of the bladder tumor (TUR-B) is performed if needed during regular follow-up. EL and TR did not show any differences based on progression of recurred low-stage bladder tumor to high stage (higher than T2) bladder tumor. Low tumor recurrence rate was observed in remnant ureter after the EL. Remnant ureterectomy was performed in only 2.1% of patients among subjects treated with the EL.
Notably, recurrent T1 bladder tumor which can be treated with only TUR-B, does not require RC. TUR-B is not an operation with as high morbidity as RC, but it is still a surgery, so it does have a certain level of morbidity. In clinical practice, many patients undergo repeated TUR-B due to multiple IVR after RNU, and repeated TUR-B is closely related to decrease in quality of life. Therefore, after RNU, the oncological outcomes, such as survival, should not be the only concern but also include the reduction of IVR. If TR reduces the IVR, this approach should be prioritized during RNU. In a number of studies, including our research, the intravesical approach was found to reduce IVR [20,21]. However, contrary results were reported in several other studies [1,7]. The reason for the contradicting outcomes between studies may be due to not considering specific conditions such as tumor location, multifocality of tumor or preoperative URS which could affect outcome. Therefore, we conducted subgroup analysis according to factors which could affect to outcome. In our results, the TR group showed significantly improved IVRFS in patients with renal pelvis tumor, with single lesion and in patients who underwent URS before RNU. In patients with ureter tumor and multiple tumors, surgeons tend to perform more TR. In the present study, all surgeons always performed TR of bladder cuff when the tumor was located in the far distal ureter which involved ureterovesical junction, which could be the reason for no significant difference in IVR rate between TR and EL when the tumor was located in the ureter. Though there is restriction to interpret these results, in the clinical practice, TR should be recommended for the patients with renal pelvis and/or single tumor as well as ureter and/or multiple lesions. Further research is needed to compare IVR between TR and EL in each specific situation.
The present study had several limitations. First, the retrospective nature of the study and non-randomized design with surgical treatment based on surgeon preference or tumor location, both introduce selection bias and are shortcomings. When considering selection bias due to tumor location, the tumor location during TR tended to be more prominent in the ureter, especially the distal ureter, but in terms of tumor location, there was no statistically significant difference between the TR and the EL group. Therefore, selection bias due to tumor location does not seem to be critical. In addition, there was no significant difference in other factors such as tumor size, pT category, grade, nodal status, resection margin status, presence of carcinoma in situ, and presence of LVI between the two groups, so the influence of selection bias due to these factors is unlikely to be large. Second, data on immediate intravesical therapy after RNU, which can have a significant effect on IVR after RNU, are not included in this study. In our center, intravesical therapy has been performed for the first time since the second half of 2018, so unfortunately, there are no data available on this. And we could not evaluate the impact of intravesical therapy on IVR after RNU. Third, the study was conducted for an extended period (1994–2018) and involved 10 different surgeons, which could also cause bias. Furthermore, subtile technical variations among surgeons in distal ureter approaches and improved techniques for BCE over the years may have confounded the overall results. Nevertheless, the research included a large sample size and extended follow-up period. According to Margulis et al. [29], UTUC recurred at a median of 10.4 months in their multicenter series of 1,363 patients [4]. Although there was wide variability in follow-up duration, the median follow-up of 37.7 months should suffice to detect most UTUC recurrences due to the natural history of the disease. Also, analysis of the bladder recurrence rate provides added value. The retrospective analysis extracted from the Surveillance, Epidemiology, and End Results database cannot provide this type of information because it is not traced [30]. In addition, the correlation between bladder cuff method and IVR was analyzed based on preoperative tumor factors, which may help surgeons select the appropriate bladder cuff method in each clinical situation.
TR of bladder cuff during RNU for UTUC was associated with improved IVRFS and OS compared with the EL. The current study confirmed that the classic complete excision of intramural portion of distal ureter should be implemented during the RNU. It is highly recommended for the surgeon to make sure that the BCE is completely performed during the RNU.
NotesEthical Statement This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. 2020-06-013). The requirement for written informed consent was waived according to the decision of IRB. Author Contributions Conceived and designed the analysis: Ryoo H, Kim J, Kang M, Jeon HG, Jeong BC, Seo SI, Jeon SS, Lee HM, Sung HH. Collected the data: Ryoo H, Kim T, Kang M, Jeon HG, Jeong BC, Seo SI, Jeon SS. Contributed data or analysis tools: Ryoo H, Kim J, Kim T, Jeong BC, Seo SI, Jeon SS, Lee HM. Performed the analysis: Ryoo H, Sung HH. Wrote the paper: Ryoo H, Sung HH. Fig. 1Oncologic outcomes after radical nephroureterectomy according to bladder cuff method: intravesical recurrence-free survival (A), cancer-specific survival (B), and overall survival (C). EL, extravesical ligation; SE, standard error; TR, transvesical resection. 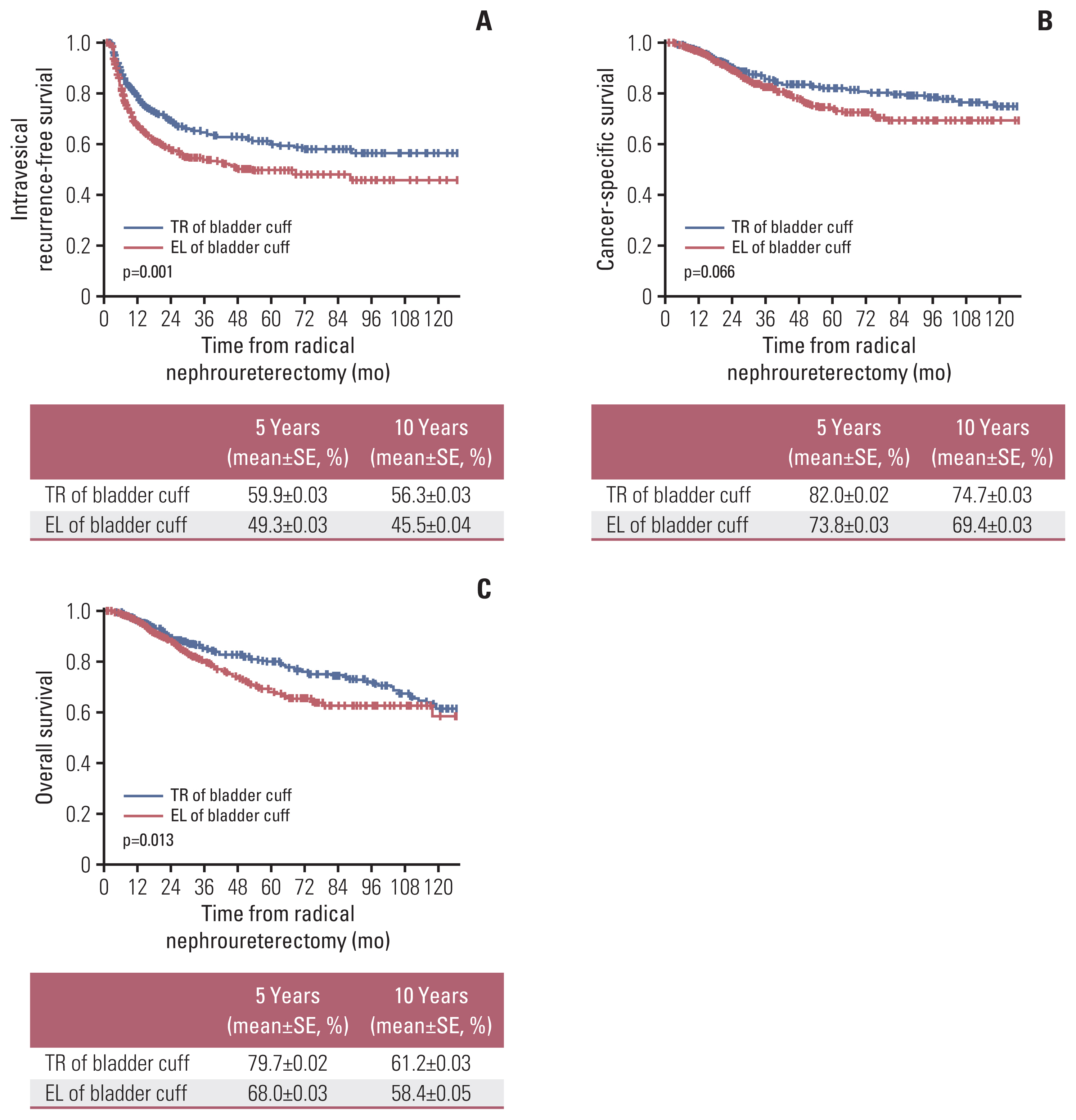
Fig. 2Radical cystectomy–free survival after radical nephroureterectomy according to bladder cuff method. EL, extravesical ligation; SE, standard error; TR, transvesical resection. 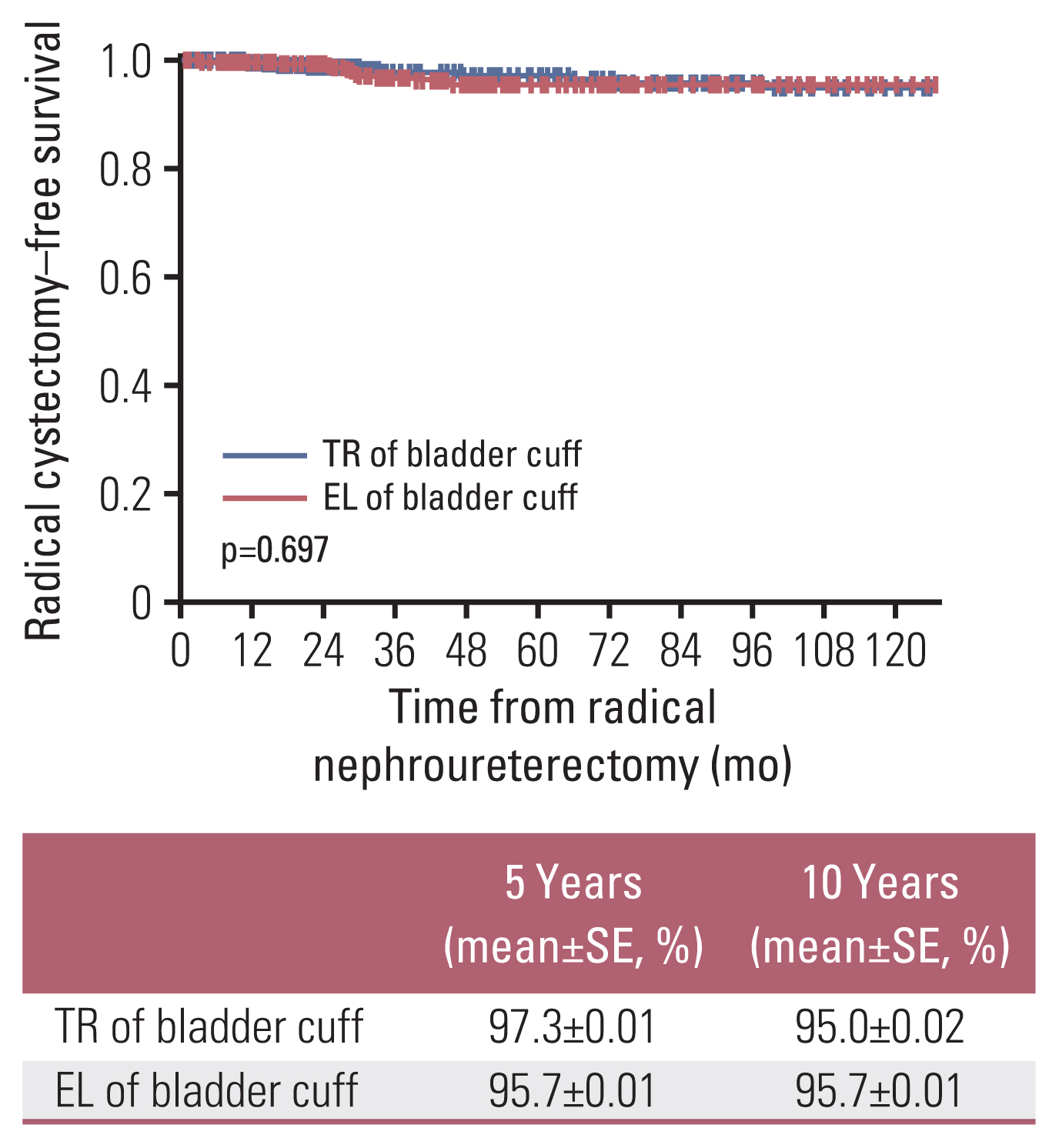
Table 1Baseline characteristics of 856 patients without previous bladder tumor history treated radical nephroureterectomy for upper tract urothelial carcinoma Table 2Multivariable Cox regression analyses predicting intravesical recurrence, CSS, and OS References1. Krabbe LM, Westerman ME, Bagrodia A, Gayed BA, Khalil D, Kapur P, et al. Surgical management of the distal ureter during radical nephroureterectomy is an independent predictor of oncological outcomes: results of a current series and a review of the literature. Urol Oncol. 2014;32:54.
2. Gupta R, Paner GP, Amin MB. Neoplasms of the upper urinary tract: a review with focus on urothelial carcinoma of the pelvicalyceal system and aspects related to its diagnosis and reporting. Adv Anat Pathol. 2008;15:127–39.
3. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5.
4. Allard CB, Alamri A, Dason S, Farrokhyar F, Matsumoto ED, Kapoor A. The method of bladder cuff excision during laparoscopic radical nephroureterectomy does not affect oncologic outcomes in upper tract urothelial carcinoma. World J Urol. 2013;31:175–81.
5. Lee SM, McKay A, Grimes N, Umez-Eronini N, Aboumarzouk OM. Distal ureter management during nephroureterectomy: evidence from a systematic review and cumulative analysis. J Endourol. 2019;33:263–73.
6. Azemar MD, Comperat E, Richard F, Cussenot O, Roupret M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol. 2011;29:130–6.
7. Xylinas E, Rink M, Cha EK, Clozel T, Lee RK, Fajkovic H, et al. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2014;65:210–7.
8. Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584–94.
9. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–71.
10. Phe V, Cussenot O, Bitker MO, Roupret M. Does the surgical technique for management of the distal ureter influence the outcome after nephroureterectomy? BJU Int. 2011;108:130–8.
11. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73:111–22.
12. Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, et al. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. 2010;57:963–9.
13. Lughezzani G, Sun M, Perrotte P, Shariat SF, Jeldres C, Budaus L, et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol. 2010;57:956–62.
14. Walton TJ, Sherwood BT, Parkinson RJ, Obakponovwe O, Thomas SA, Taylor MC, et al. Comparative outcomes following endoscopic ureteral detachment and formal bladder cuff excision in open nephroureterectomy for upper urinary tract transitional cell carcinoma. J Urol. 2009;181:532–9.
15. Romero FR, Schaeffer EM, Muntener M, Trock B, Kavoussi LR, Jarrett TW. Oncologic outcomes of extravesical stapling of distal ureter in laparoscopic nephroureterectomy. J Endourol. 2007;21:1025–7.
16. Ko R, Chew BH, Hickling DR, Razvi H, Luke PP, Chin JL, et al. Transitional-cell carcinoma recurrence rate after nephroureterectomy in patients who undergo open excision of bladder cuff v transurethral incision of the ureteral orifice. J Endourol. 2007;21:730–4.
17. Matin SF, Gill IS. Recurrence and survival following laparoscopic radical nephroureterectomy with various forms of bladder cuff control. J Urol. 2005;173:395–400.
18. Saika T, Nishiguchi J, Tsushima T, Nasu Y, Nagai A, Miyaji Y, et al. Comparative study of ureteral stripping versus open ureterectomy for nephroureterectomy in patients with transitional carcinoma of the renal pelvis. Urology. 2004;63:848–52.
19. Salvador-Bayarri J, Rodriguez-Villamil L, Imperatore V, Palou Redorta J, Villavicencio-Mavrich H, Vicente-Rodriguez J. Bladder neoplasms after nephroureterectomy: does the surgery of the lower ureter, transurethral resection or open surgery, influence the evolution? Eur Urol. 2002;41:30–3.
20. Lai S, Guo R, Seery S, Wu P, Liu J, Zhang Y, et al. Assessing the impact of different distal ureter management techniques during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma on oncological outcomes: a systematic review and meta-analysis. Int J Surg. 2020;75:165–73.
21. Kapoor A, Dason S, Allard CB, Shayegan B, Lacombe L, Rendon R, et al. The impact of method of distal ureter management during radical nephroureterectomy on tumour recurrence. Can Urol Assoc J. 2014;8:E845–52.
22. McCarron JP, Mills C, Vaughn ED Jr. Tumors of the renal pelvis and ureter: current concepts and management. Semin Urol. 1983;1:75–81.
23. Kakizoe T, Fujita J, Murase T, Matsumoto K, Kishi K. Transitional cell carcinoma of the bladder in patients with renal pelvic and ureteral cancer. J Urol. 1980;124:17–9.
25. Bloom NA, Vidone RA, Lytton B. Primary carcinoma of the ureter: a report of 102 new cases. J Urol. 1970;103:590–8.
26. Strong DW, Pearse HD. Recurrent urothelial tumors following surgery for transitional cell carcinoma of the upper urinary tract. Cancer. 1976;38:2173–83.
27. Strong DW, Pearse HD, Tank ES Jr, Hodges CV. The ureteral stump after nephroureterectomy. J Urol. 1976;115:654–5.
28. Sung HH, Jeon HG, Han DH, Jeong BC, Seo SI, Lee HM, et al. Diagnostic ureterorenoscopy is associated with increased intravesical recurrence following radical nephroureterectomy in upper tract urothelial carcinoma. PLoS One. 2015;10:e0139976.
|
|