AbstractPurposeThis study aimed to investigate the impact of BRCA1/2 mutational status on survival outcomes in patients with platinum-sensitive relapsed (PSR) epithelial ovarian cancer (EOC).
Materials and MethodsWe retrospectively identified patients who received secondary treatment for PSR EOC at our institution between January 2007 and June 2021 and who underwent BRCA1/2 gene testing by either germline or somatic methods. The association between BRCA1/2 mutational status and survival outcomes was evaluated. Both secondary cytoreductive surgery (CRS) and maintenance therapy were stratified considering real-world clinical practice.
ResultsOf 262 patients, 91 (34.7%) and 171 (65.3%) were assigned to BRCA1/2 mutation and wild-type groups, respectively. The two groups had similar proportions of patients undergoing secondary CRS (26.4% vs. 32.7%, p=0.286) and maintenance therapy (54.9% vs. 46.2%, p=0.178). Overall, no differences in progression-free survival (PFS; median, 19.7 vs. 15.1 months, p=0.120) and overall survival (OS; p=0.400) were observed between the two groups. In multivariate analyses, BRCA1/2 mutational status was not associated with PFS (adjusted hazard ratio, 0.816; 95% confidence interval, 0.596 to 1.119; p=0.207). BRCA1/2 mutational status did not affect PFS among patients who underwent secondary CRS (n=80) and among those who did not (n=182) (p=0.074 and p=0.222, respectively). PFS did not differ in the BRCA1/2 mutational status among the patients who received bevacizumab maintenance (n=90, p=0.992).
IntroductionOvarian cancer, the most fatal gynecologic malignancy, is one of the leading causes of female cancer deaths in Korea [1]. In conjunction with a western lifestyle and socio-cultural change, such as delayed and non-marriage and having fewer children, the incidence of ovarian cancer has gradually increased in Korea [2]. Approximately 90% of all ovarian cancer cases are histologically epithelial ovarian cancer (EOC), which tends to be diagnosed at an advanced stage and relapses despite primary treatment, consisting of extensive cytoreductive surgery (CRS) and platinum-based combination chemotherapy [3]. Treatment of recurrent EOC is challenging since chemoresistance acquisition is responsible for the treatment failure [4].
Traditionally, retreatment of platinum-based combination chemotherapy has been recommended as a secondary treatment to patients with platinum-sensitive recurrence (PSR), defined as ≥ 6 months of platinum-free interval (PFI) [5,6]. However, recent landmark phase III randomized controlled trials (RCTs) proved survival benefit from maintenance therapy with poly(ADP-ribose) polymerase inhibitors (PARPis), both olaparib (OLA) and niraparib (NIRA), which significantly improved the progression-free survival (PFS) in PSR EOC, especially for BRCA mutated, high-grade serous or endometrioid carcinoma [7–9]. OLA maintenance even resulted in prolonged overall survival (OS) [10]. Based on these promising data, more and more patients with EOC are expected to receive germline and/or somatic BRCA1/2 gene testing.
Before the advent of PARPis, mutations in the BRCA1 and BRCA2 genes received considerable attention by researchers since they are associated with increased risk of developing breast and ovarian cancers [11] and demonstrate a favorable prognosis in primary EOC, probably resulting from better response to platinum-based chemotherapy [12,13]. Nowadays, BRCA1/2 gene testing is conducted to screen patients for PARPi maintenance therapy.
Meanwhile, other therapeutic options are also available for the secondary treatment of PSR EOC. Previous phase III RCTs [14,15] demonstrated a PFS benefit from concomitant use with platinum-based doublets and maintenance of bevacizumab (BEV), a humanized anti-vascular endothelial growth factor monoclonal antibody, in PSR EOC. In 2021, the Gynecologic Cancer InterGroup, 6th Ovarian Cancer Consensus Conference stated that secondary CRS should be considered in all patients with recurrent disease fulfilling criteria predictive of successful complete resection, based on the recently published phase III trials, DESKTOP-III [16] and SOC-1 [17], which confirmed the survival benefit from secondary CRS.
To date, the survival impact of germline/somatic BRCA1/2 mutations in PSR EOC is relatively less investigated than those in primary EOC. Moreover, there are still unanswered questions surrounding maintenance therapy and secondary CRS. For example, it remains unknown whether efficacy of BEV or survival benefit from secondary CRS differs according to BRCA1/2 mutational status or not. The maintenance strategies to be administered following secondary CRS according to the residual disease and individual patients’ BRCA1/2 mutational status have not been established [18].
Thus, we aimed to investigate impact of BRCA1/2 mutational status on the survival outcomes in patients with PSR EOC. Both secondary CRS and maintenance therapy were stratified considering real-world clinical practice. For this purpose, we decided to present our institution’s experience on the management of PSR EOC.
Materials and Methods1. Study populationFrom the institution’s Ovarian Cancer Cohort, we identified consecutive patients with EOC who met the following conditions: (1) aged more than 18 years at the time of initial diagnosis, (2) those who had undergone both CRS and platinum-based chemotherapy as first-line treatment, (3) those who experienced the first relapse between January 2007 and June 2021 with ≥ 6 months of PFI, and (4) those whose germline and/or somatic BRCA1/2 mutational status were available. However, we excluded patients if (1) they had brain metastases at the time of disease recurrence, (2) received non-platinum-based second-line chemotherapy, (3) received chemotherapy prior to secondary CRS, or (4) had insufficient clinical data or lost to follow-up during or immediately after completion of the secondary treatment.
At our institution, any specific patient selection criteria or models, such as Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) score [19] or international model (iMO-DEL) [17], have not been used for predicting the feasibility of complete gross resection. Instead, physicians suggested secondary CRS to patients with PSR EOC when complete resection was amenable based on an integrative judgment considering PFI, performance status, and imaging studies, including 18F-fluoro-deoxy-glucose positron emission tomography/computed tomography (PET/CT). In this aspect, we excluded three patients enrolled in DESKTOP-III [16], in which a positive AGO score was the principal inclusion criterion.
Meanwhile, our institution was one of the predominant academic centers that actively enrolled patients with PSR EOC in GOG-213 trial [15,20]. Of our study population, 29 patients participated in that trial. All these patients were the candidate of secondary CRS, and through the randomization, underwent the secondary treatment as follows: secondary CRS plus BEV maintenance (n=8); secondary CRS without any maintenance (n=4); and no surgery, but BEV maintenance (n=16); and no surgery, no maintenance (n=1).
2. Secondary treatment and surveillanceIn this study, all the patients underwent BRCA1/2 gene testing at Seoul National University Hospital by either germline or somatic methods as described in our previous study [13]. Since February 2016, germline BRCA1/2 gene testing method has been revised from direct sequencing to next-generation sequencing (NGS). For somatic BRCA1/2 gene testing, the institution’s own NGS cancer panel was applied to archival formalin-fixed paraffin-embedded tumor tissues. According to the recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [21], patients who had “pathogenic” and “likely pathogenic” variants in germline or somatic BRCA1 and BRCA2 were regarded as the BRCA mutation group. Meanwhile, the rest of the patients were regarded as the BRCA wild-type group.
Physicians recommend second-line chemotherapy and maintenance therapy to patients as per the practice guidelines of Korean Society of Gynecologic Oncology [22]. Those who completed second-line chemotherapy without BEV belonged to one of the three indices: no maintenance, OLA maintenance, and NIRA maintenance (rucaparib was excluded since it is not available in Korea). Responders to the chemotherapy had the opportunity to undergo OLA (capsules as 400 mg or tablets as 300 mg twice a day orally) or NIRA (capsules as 200 mg or 300 mg once daily orally) in the presence of BRCA mutated, high-grade serous carcinoma. Despite the absence of BRCA1/2 mutations, two and three patients with high-grade serous carcinoma received OLA (tablets as 300 mg twice a day orally) and NIRA (capsules as 200 mg or 300 mg once daily orally), respectively. Dose reduction and interruption was permitted at the physicians’ discretion.
Meanwhile, patients who received BEV concomitantly with chemotherapy belonged to one of the two regimens: (1) paclitaxel (175 mg/m2 of body surface area)+carboplatin (area under the curve 5)+BEV (15 mg/kg) intravenously every 3 weeks, or (2) gemcitabine (1,000 mg/m2 of body surface area) on days 1 and 8 +carboplatin (area under the curve 4)+BEV (15 mg/kg) intravenously every 3 weeks. If secondary CRS was performed, BEV was started on cycle 2. Following the completion of second-line chemotherapy, some patients stopped BEV, while the others continued BEV (15 mg/kg) intravenously every 3 weeks as maintenance therapy. For statistical purpose, we considered the latter as the BEV maintenance group in this study.
Maintenance therapy was continued until unacceptable toxicity, patient refusal, or disease progression, which was ascertained by computed tomography (CT) scans as per the Response Evaluation Criteria in Solid Tumors ver. 1.1 [23]. In terms of the surveillance, patients underwent CT after the third cycles of second-line chemotherapy. During BEV maintenance, CT scans were conducted every three cycles, while every 3 months during PARPi maintenance. Those who did not receive any maintenance or completed maintenance underwent routine CT scans every 3 months for the first 2 years. Thereafter, CT scans were performed every 4–6 months for the next 2 years and annually afterward.
3. Data collection and statistical analysisWe collected the patients’ clinicopathologic characteristics, including age at initial diagnosis, histologic type, International Federation of Gynecology and Obstetrics (FIGO) stage, and residual tumor size following CRS and PFIs. We also collected characteristics at the first relapse, such as age, serum cancer antigen 125 (CA-125) levels, retrospectively estimated AGO score, and details of the secondary treatment. PFS and OS were defined as the time interval from the start date of the secondary treatment to the date of disease progression and cancer-related death or the end of the study, respectively.
For comparing the patient characteristics between the two groups (e.g., BRCA mutation and wild-type groups), we used the Student’s t- or Mann-Whitney U tests for the continuous variables, while we used Pearson’s chi-squared or Fisher exact tests for the categorical variables. Kaplan-Meier methods with the log-rank test were conducted for the survival analyses. In multivariate analysis, we used a Cox proportional hazards model and calculated the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). All these statistical analyses were conducted using IBM SPSS Statistics software ver. 25.0 (IBM Corp., Armonk, NY). A p-value < 0.05 was considered as statistically significant.
Results1. Overall findingsIn total, 262 patients were included, and 91 (34.7%) and 171 (65.3%) were assigned to the BRCA mutation and wild-type groups, respectively. In the BRCA mutation group, 70 (76.9%) and 21 (23.1%) patients had mutated BRCA1 and BRCA2 genes, respectively. None of the patients had BRCA1 and BRCA2 mutations simultaneously. Detailed BRCA1/2 gene test results are summarized in S1 Table.
The patients’ clinicopathologic characteristics are presented in Table 1. The most common histologic type was high-grade serous carcinoma (83.2%), and advanced-stage disease (FIGO stage III–IV) was observed in 86.6% of the patients. Between the BRCA mutation and wild-type groups, no differences were observed in the patient’s age at initial diagnosis, FIGO stage, and the proportion of high-grade serous carcinoma. Similar proportion of patients received neoadjuvant chemotherapy between the BRCA mutation and wild-type groups (31.9% vs. 27.5%, p=0.457). The median number of neoadjuvant chemotherapy cycles was 3 in both groups (p=0.716). Through the primary CRS or interval CRS, similar proportion of patients achieved complete resection (70.3% vs. 70.8%, p=0.942). No difference in the use of BEV during primary treatment was observed between the two groups (9.9% vs. 9.4%, p=0.889). Notably, none of the patients received PARPi as primary maintenance therapy.
At the time of PSR, 80 patients (30.5%) underwent secondary CRS with 82.5% of the complete resection rate. Maintenance therapy was administered in 129 (49.2%) patients: 90 (34.4%) and 39 (14.9%) patients received BEV and PARPi maintenance, respectively; none of them received dual (BEV plus PARPi) maintenance therapy. S2 Fig. depicts the composition of the study population based on the BRCA1/2 mutational status, secondary CRS, and maintenance therapy.
The BRCA mutation and wild-type groups had similar PFI, serum CA-125 levels, and proportion of patients having a positive AGO score (Table 2). Considering the secondary treatment, the two groups showed similar proportion of patients who underwent secondary CRS (26.4% vs. 32.7%; p=0.286) and complete resection (91.7% [22/24] vs. 78.6% [44/56], p=0.209). The most frequently administered second-line chemotherapy regimen was paclitaxel-carboplatin (49.5%) in the BRCA mutation group and paclitaxel-carboplatin-BEV (46.2%) in the BRCA wild-type group.
After completion of combination chemotherapy, similar proportion of patients received maintenance therapy between the BRCA mutation and wild-type groups (54.9% vs. 46.2%, p=0.178) (Table 2). However, the maintenance drugs used were different by the BRCA1/2 mutational status. In the BRCA mutation group, PARPis were more commonly used than BEV. In the BRCA wild-type group, only five patients received PARPi maintenance, while most of the patients received BEV maintenance (n=74).
During a median observation period of 27.0 months, 191 patients (72.9%) relapsed and 39 patients (14.9%) died of the disease. Overall, the BRCA mutation and wild-type groups showed similar PFS (median, 19.7 vs. 15.1 months; p=0.120) and OS (3-year survival rate, 85.2% vs. 88.2%; p=0.400) (Fig. 1A and B). There was no difference in PFS (p=0.226) and OS (p=0.629) between the BRCA1 mutation, BRCA2 mutation, and BRCA wild-type groups (Fig. 1C and D).
Multivariate analyses adjusted for the clinicopathologic factors revealed that BRCA mutation was not associated with PFS (aHR, 0.816; 95% CI, 0.596 to 1.119; p=0.207) (Table 3) and OS (aHR, 1.376; 95% CI, 0.721 to 2.628; p=0.333) (S3 Table). Meanwhile, PFI ≥ 12 months (aHR, 0.571; 95% CI, 0.421 to 0.773; p < 0.001), secondary CRS (aHR, 0.566; 95% CI, 0.397 to 0.807; p=0.002), and maintenance therapy (aHR, 0.489; 95% CI, 0.357 to 0.669; p < 0.001) were identified as favorable prognostic factors for PFS (Table 3). Of these three factors, only secondary CRS was associated with improved OS (aHR, 0.424; 95% CI, 0.183 to 0.987; p=0.046) (S3 Table).
2. Secondary CRS analysisThe comparison between the patient characteristics of the secondary CRS (n=80) and no surgery groups (n=182) are presented in S4 Table. There was no difference in BRCA1/2 mutational status (p=0.234) between the two groups; however, patients in the secondary CRS group had longer PFI (median, 22.8 vs. 12.3 months; p < 0.001), and were younger at the time of PSR (p=0.022) than those in the no surgery group. The secondary CRS group had lower serum CA-125 levels (median, 62.3 vs. 112.4 IU/mL; p < 0.001) but had a higher proportion of patients with a positive AGO score (76.3% vs. 55.5%, p=0.001). Similar proportion of patients received maintenance therapy (42.5% vs. 52.2%, p=0.148) between the secondary CRS and no surgery groups. Maintenance with BEV and PARPi were also similarly administered (p=0.272).
In the survival analysis, the secondary CRS group showed significantly improved PFS than the no surgery group (median, 23.2 vs. 13.9 months; p < 0.001) (Fig. 2A). Such a PFS benefit from secondary CRS was consistent, regardless of BRCA1/2 mutational status (Fig. 2B and C). Investigating the impact of BRCA1/2 mutational status among patients who underwent secondary CRS and among those who did not undergo surgery, we found that BRCA1/2 mutational status did not affect the patients’ PFS (p=0.074 and p=0.222, respectively) (Fig. 2D).
The secondary CRS group showed significantly improved OS than the no surgery group (3-year survival rate, 93.0% vs. 84.0%; p=0.026). However, among patients who underwent secondary CRS and among those who did not undergo surgery, BRCA1/2 mutational status did not affect the patients’ OS (p=0.774 and p=0.454, respectively) (S5 Fig.).
We subsequently split the secondary CRS group into complete resection (n=66) and residual tumor subgroups (n=14). The complete resection subgroup showed significantly better PFS (median, 23.4 vs. 13.9 months; p < 0.001) and OS (3-year survival rate, 97.3% vs. 84.0%; p=0.005), compared to the no surgery group. In contrast, no differences in PFS (p=0.241) and OS (p=0.561) were observed between the residual tumor subgroup and no surgery group (S6 Fig.). Owing to the small sample size of the residual tumor subgroup, we could not conduct subsequent survival analyses of BRCA1/2 mutational status.
3. Maintenance therapy analysisAlthough there was no difference in OS (p=0.582), PARPi maintenance (median, 23.7 vs. 14.5 months; p=0.001) and BEV maintenance (median, 17.2 vs. 14.5 months; p=0.012) showed significantly better PFS, compared to the no maintenance group (S7A and S7B Fig.). Multivariate analysis adjusting PFI, CA-125, and secondary CRS revealed that both BEV (aHR, 0.558; 95% CI, 0.396 to 0.788; p=0.001) and PARPi (aHR, 0.398; 95% CI, 0.243 to 0.651; p < 0.001) significantly improved the patients’ PFS compared to no maintenance. Meanwhile, we found that BRCA1/2 mutational status did not affect PFS of the patients who received BEV maintenance (n=90, p=0.992) and PFS of those without any maintenance (n=133, p=0.101) (S7C and S7D Fig.).
In the BRCA mutation group, PARPi users had better PFS than those who did not receive any maintenance therapy with marginal significance (median, 21.8 vs. 19.7 months; p=0.054), whereas BEV users did not (p=0.578). In the BRCA wild-type group, both PARPi users (p=0.033) and BEV users (p=0.007) showed significantly improved PFS, compared to those without maintenance (Fig. 3A and B).
In the secondary CRS group, the median PFS for PARPi users was 43.2 months, which was significantly better than 28.4 months for BEV users (p=0.038) and 19.7 months for those without maintenance (p=0.009). No difference in PFS was observed between BEV and no maintenance (p=0.238). In the no surgery group, both the PARPi (p=0.007) and BEV users (p=0.012) had significantly improved PFS, compared to those without maintenance. However, no difference in PFS was observed between PARPi and BEV (p=0.585) (Fig. 3C and D).
Lastly, we investigated the impact of maintenance therapy based on the BRCA1/2 mutational status exclusively among patients who underwent secondary CRS. When the BRCA mutation was identified (n=24), PARPi maintenance showed better PFS with marginal significance, compared to those who did not receive PARPi maintenance (median, 43.2 vs. 23.2 months; p=0.058). Meanwhile, in the BRCA wild-type cases (n=56), BEV users and non-users had similar PFS (median, 27.5 vs. 18.4 months; p=0.253) (S8 Fig.).
DiscussionThis real-world evidence study suggests that germline/somatic BRCA1/2 mutational status itself might not be associated with PFS and OS in PSR EOC. In contrast, secondary CRS was identified as a favorable prognostic factor for PFS and OS, while maintenance therapy was associated with improved PFS. Whether the patients received secondary CRS or not, BRCA1/2 mutational status did not affect the patients’ prognosis. Among the patients who received BEV maintenance, PFS did not differ in the BRCA1/2 mutational status.
In literature, studies investigating the impact of BRCA1/2 mutational status on the survival outcomes in EOC has been mainly conducted in primary settings [12,13,24,25]. Previously, our research team reported that patients harboring BRCA1/2 mutations demonstrated better PFS than those who did not [13]. Several years have passed since then, and PARPis are widely used in newly diagnosed and PSR EOC. Nowadays, it is not easy to conduct studies to identify the pure effect of BRCA1/2 mutational status on the prognosis, excluding the use of PARPi maintenance. Therefore, we conducted patient stratification based on several factors, including maintenance therapy.
Consistent with previous phase III RCTs, OCEANS [14] and GOG-213 [15], we observed significantly better PFS from BEV maintenance, rather than no maintenance, in PSR EOC. While both trials did not report the proportion of patients with BRCA1/2 mutations, the current study found similar PFS between BRCA mutated BEV users and BRCA wild-type BEV users. Similarly, GOG-218 demonstrated that germline/somatic BRCA1/2 mutations were not predictive of BEV activity, however, the study only included newly diagnosed advanced EOC [26].
In our study, no difference in PFS was observed between BEV maintenance and no maintenance among the patients with BRCA mutated PSR EOC. Similar results were also reported in Lorusso et al.’s multi-center retrospective study: among the patients with BRCA mutated, newly diagnosed advanced high-grade serous ovarian cancer, there were no differences in PFS and OS between BEV users (n=58) and non-users (n=90) [27]. In contrast, BEV maintenance significantly improved PFS than no maintenance in BRCA wild-type PSR EOC, which was also similar to Lorusso et al.’s study [27]. However, our study and Lorusso et al.’s study differed in disease settings and histologic subtypes (PSR EOC vs. newly diagnosed advanced high-grade serous ovarian cancer). Nevertheless, as a possible explanation, we could infer that the intrinsically increased response to chemotherapy of BRCA mutated tumors seems to exceed the BEV’s anti-angiogenic effects and ability to change the tumor microenvironment in terms of morphology, functions, and permeability [12,13,28,29]. Interestingly, the survival benefit from BEV maintenance in BRCA wild-type PSR EOC disappeared when patients received secondary CRS. Although this could be owing to the small sample size; nevertheless, we hypothesize that the survival benefit from secondary CRS might further exceed those from BEV.
To our knowledge, there are only three phase III RCTs, which investigated the survival outcomes of secondary CRS in PSR EOC [16,17,20]. While GOG-213 failed to prove survival benefit from secondary CRS [20], DESKTOP-III proved that secondary CRS followed by chemotherapy significantly increases PFS and OS compared to chemotherapy alone [16]. SOC-1 also proved that secondary CRS was associated with a significantly longer PFS [17]. A recent meta-analysis study of 36 studies with 2,805 patients also reported significantly prolonged OS by secondary CRS [30]. In our study, we also observed an association between secondary CRS and improved PFS and OS. Especially, PFS benefit from the secondary CRS was profound among the patients who achieved complete resection. Some might argue that a validated criteria predictive of successful complete resection was not used in our study. It is true that secondary CRS was determined by physicians’ discretion; nevertheless, by referring to multiple factors, including the patient factors and PET/CT, the complete resection rate at our institution reached 80.5%, which is quite higher than those of the three phase III RCTs (67%–77%) [16,17,20].
Moreover, while the study populations of the three phase III RCTs lack information on the BRCA1/2 mutational status and only few (less than 10%) received PARPi maintenance [31], our study has the advantage of including this information and a higher proportion of patients with PARPi maintenance (approximately 15%). Both in the secondary CRS group and no surgery group, BRCA1/2 mutational status did not affect the PFS in our study. Survival benefit from surgical resection of cancer stem cells and chemoresistant tumors seems to exceed intrinsically increased chemotherapy res-ponses from BRCA1/2 mutations [32]. Therefore, secondary CRS aimed at complete resection should be considered a top priority in managing patients with PSR EOC, regardless of BRCA1/2 mutational status. Furthermore, patients with BRCA1/2 mutations who underwent secondary CRS showed a trend of improved PFS when PARPi maintenance was administered. The small sample size of our study population might produce such statistical insignificance. Nevertheless, considering our study results and those from Marchetti et al.’s retrospective matched cohort study [33], PARPi maintenance therapy after secondary CRS and platinum-based chemotherapy might be a promising treatment strategy for resectable, BRCA mutated PSR EOC. Further large prospective cohort studies are warranted.
Our study has several limitations. First, small sample size, especially for each maintenance therapy, was the most problematic. Although we conducted further subgroup analyses, many analyses were underpowered and inconclusive. In addition, owing to the small sample size, we could not analyze survival outcomes by the specific sites of BRCA1/2 mutations, such as the ovarian cancer cluster region, which Ha et al. recently investigated [34]. Second, selection bias, which inherently originated from the retrospective study design, was also a major deterrent. For example, physicians might have selected more favorable cases as candidates for secondary CRS. Third, homologous recombination deficiency (HRD) was not tested in our study population; therefore, the impact of the HRD status, beyond the BRCA1/2 mutational status, on the survival outcomes still remain unknown. Fourth, the adverse events from chemotherapy and maintenance therapy, actual period of maintenance therapy, and dose reduction/interruption were not investigated in the current study. Fourth, the cost-effectiveness and quality of life assessment were not conducted. Lastly, Korea’s specific sociomedical environment is emulated in the study results. For example, the first PARPi (OLA) was approved as first-line maintenance therapy in BRCA mutated, high-grade serous ovarian cancer in October 2019; thus, none of the study population received PARPi as first-line maintenance therapy.
In conclusion, our real-world evidence study demonstrates that BRCA1/2 mutational status itself was not associated with PFS and OS in PSR EOC. Among the patients who received BEV maintenance therapy, PFS did not differ in the BRCA1/2 mutational status. PFS benefit from the secondary CRS was consistently observed, regardless of BRCA1/2 mutational status. Therefore, secondary CRS aimed at complete resection should be prioritized, regardless of BRCA1/2 mutational status. For patients with BRCA1/2 mutations, PARPi maintenance therapy might confer additional PFS benefit after secondary CRS. Our study results could be useful in patient counselling and implementing individualized treatment for patients with PSR EOC.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The Institutional Review Board of the Seoul National University Hospital (SNUH) approved this retrospective cohort study (No. H-2106-007-1233). The study was performed in accordance with the principles of the Declaration of Helsinki, and the requirement for informed consent was waived. Author Contributions Conceived and designed the analysis: Kim SI, Lee M. Collected the data: Kim SI, Lim H, Lee M. Contributed data or analysis tools: Kim SI, Lim H, Chung HH, Kim JW, Park NH, Song YS, Lee M. Performed the analysis: Kim SI, Lee M. Wrote the paper: Kim SI, Lim H, Kim HS, Chung HH, Kim JW, Park NH, Song YS, Lee M. Lee M. AcknowledgmentsThis work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean government (the Ministry of Science and ICT; No. NRF-2020R1G1A1005711), and the Korea Medical Device Development Fund grants, funded by the Korean government: the Ministry of Trade, Industry and Energy (No. 1711137954, RS-2020-KD000028); and the Ministry of Science and ICT (No. 1711135020, RS-2020-KD000106).
Fig. 1Comparisons of survival outcomes according to the BRCA1/2 mutational status: (A, C) progression-free survival, (B, D) overall survival. CI, confidence interval. 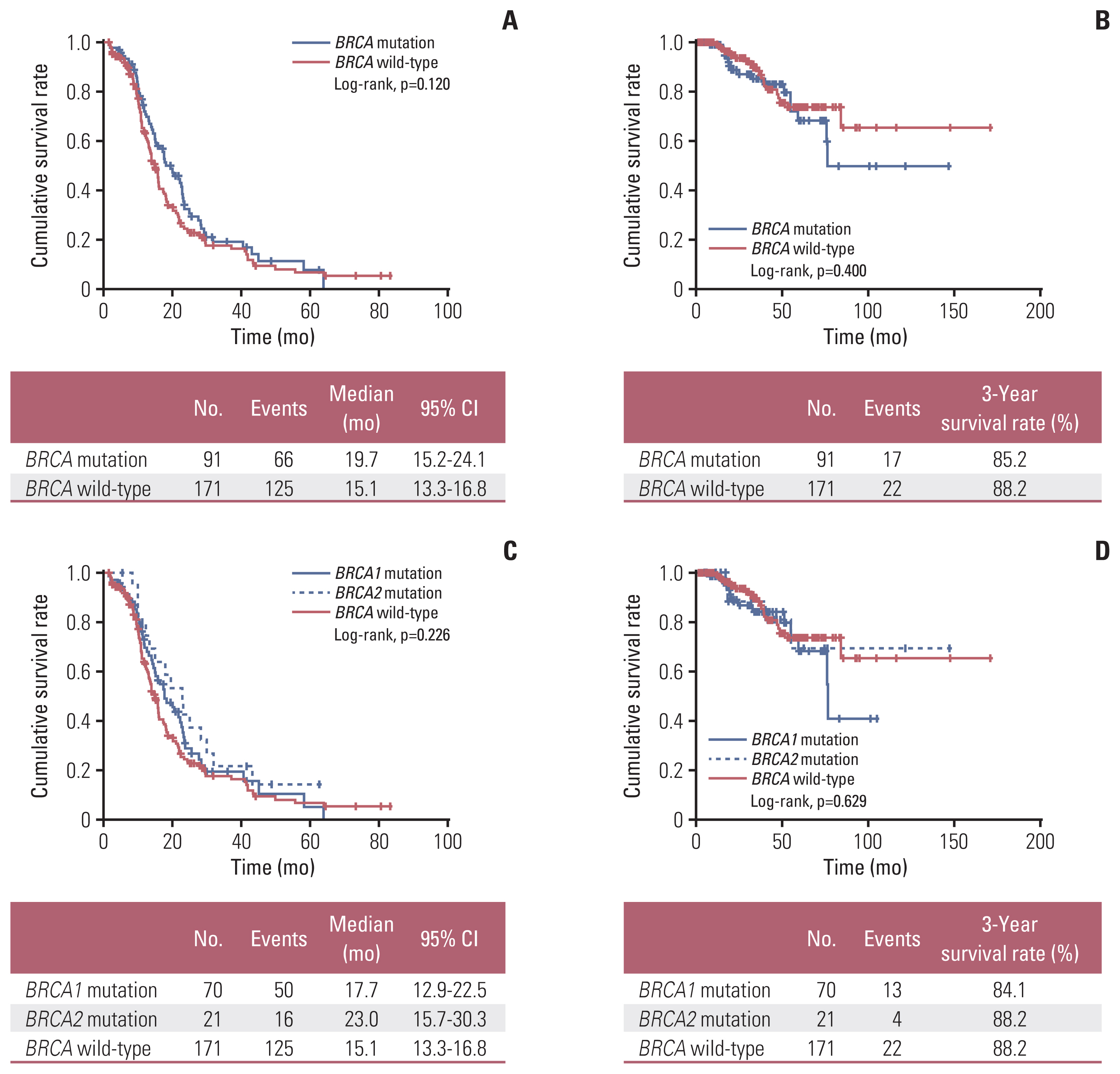
Fig. 2Comparisons of progression-free survival based on secondary cytoreductive surgery. (A) All patients. (B) BRCA mutation group. (C) BRCA wild-type group. (D) Combinations of secondary cytoreductive surgery and BRCA1/2 mutational status. CI, confidence interval; CRS, cytoreductive surgery. 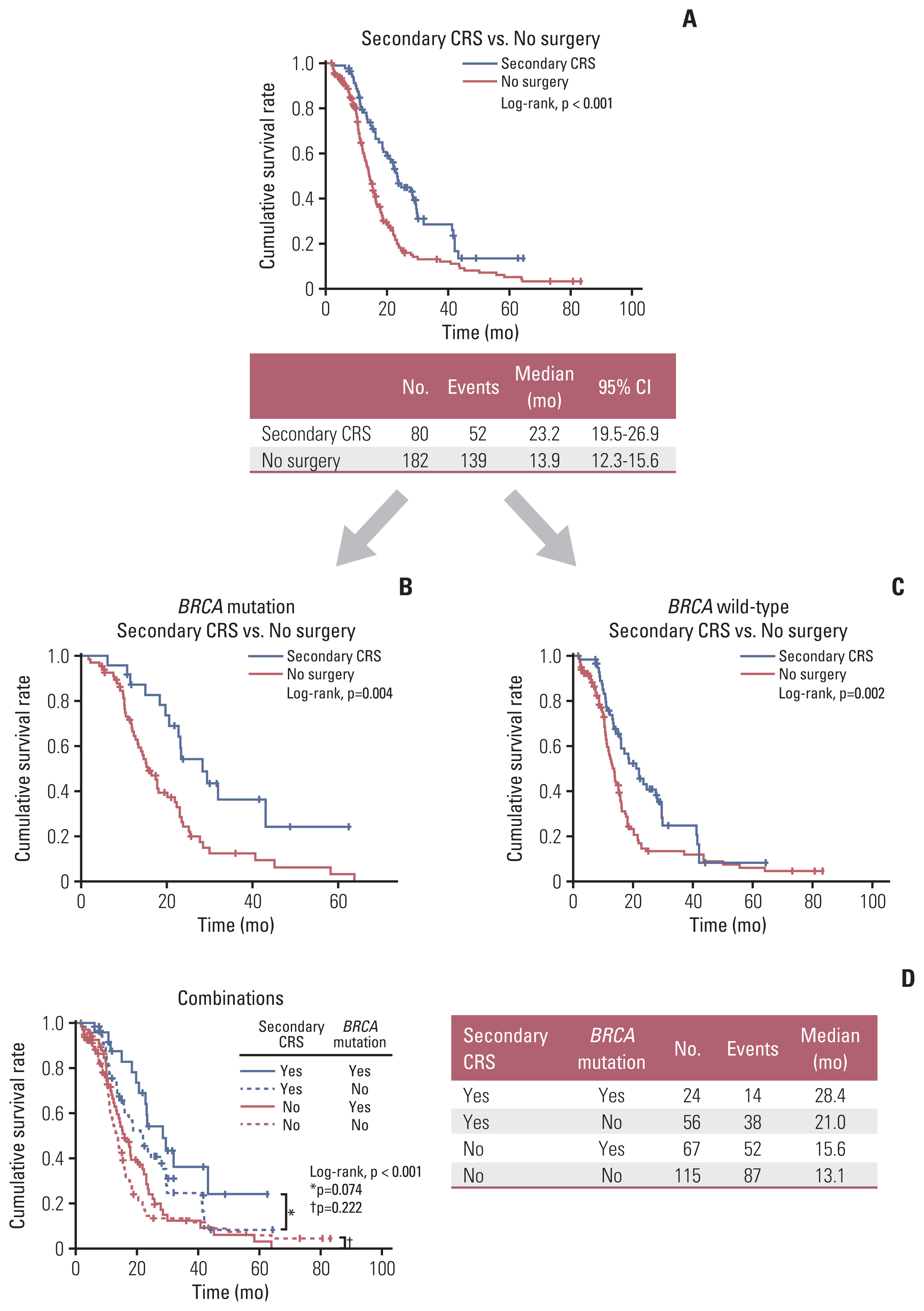
Fig. 3Comparisons of progression-free survival according to the maintenance therapy by BRCA1/2 mutational status (A, B) and by secondary cytoreductive surgery (CRS) (C, D). BEV, bevacizumab; CI, confidence interval; PARPi, poly(ADP-ribose) polymerase inhibitor. 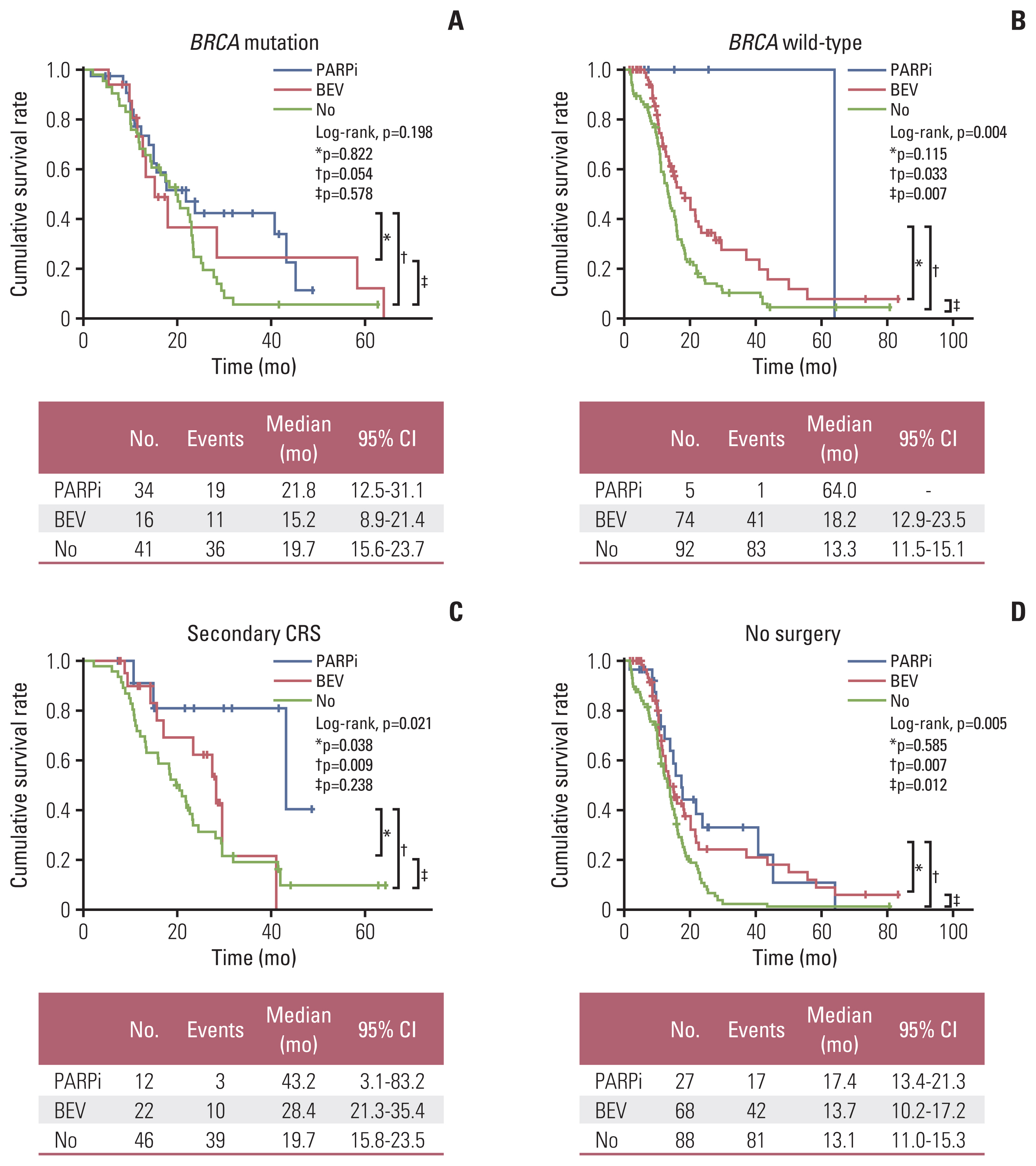
Table 1Patients’ characteristics at the time of primary treatment Table 2Patients’ characteristics at the time of recurrence and during secondary treatment
Value are presented as number (%) unless otherwise indicated. AGO, Arbeitsgemeinschaft Gynäkologische Onkologie; BEV, bevacizumab; CA-125, cancer antigen 125; CRS, cytoreductive surgery; NIRA, niraparib; OLA, olaparib; PARPi, poly(ADP-ribose) polymerase inhibitor; PLD, pegylated liposomal doxorubicin; SD, standard deviation. Table 3Factors associated with progression-free survival References1. Jung KW, Won YJ, Kang MJ, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res Treat. 2022;54:345–51.
2. Lim MC, Won YJ, Ko MJ, Kim M, Shim SH, Suh DH, et al. Inci-dence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol. 2019;30:e38.
4. Kim S, Han Y, Kim SI, Kim HS, Kim SJ, Song YS. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis Oncol. 2018;2:20.
5. National Comprehensive Cancer Network. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1, 2022 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022. [cited 2022 May 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
6. Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol. 2019;30:672–705.
7. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92.
8. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64.
9. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84.
10. Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:620–31.
11. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
12. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6.
13. Kim SI, Lee M, Kim HS, Chung HH, Kim JW, Park NH, et al. Germline and somatic BRCA1/2 gene mutational status and clinical outcomes in epithelial peritoneal, ovarian, and fallopian tube cancer: over a decade of experience in a single institution in Korea. Cancer Res Treat. 2020;52:1229–41.
14. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45.
15. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91.
16. Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123–31.
17. Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439–49.
18. Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open. 2021;6:100149.
19. Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21:289–95.
20. Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929–39.
21. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
22. Suh DH, Chang SJ, Song T, Lee S, Kang WD, Lee SJ, et al. Practice guidelines for management of ovarian cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol. 2018;29:e56.
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
24. Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012;118:3703–9.
25. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
26. Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–28.
27. Lorusso D, Marchetti C, Conte C, Giudice E, Bolomini G, Vertechy L, et al. Bevacizumab as maintenance treatment in BRCA mutated patients with advanced ovarian cancer: a large, retrospective, multicenter case-control study. Gynecol Oncol. 2020;159:95–100.
28. Vencken P, Kriege M, Hoogwerf D, Beugelink S, van der Burg MEL, Hooning MJ, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22:1346–52.
29. Fuso Nerini I, Cesca M, Bizzaro F, Giavazzi R. Combination therapy in cancer: effects of angiogenesis inhibitors on drug pharmacokinetics and pharmacodynamics. Chin J Cancer. 2016;35:61.
30. Baek MH, Park EY, Ha HI, Park SY, Lim MC, Fotopoulou C, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer: a meta-analysis. J Clin Oncol. 2022;40:1659–70.
31. Lee YY, Choi MC, Park JY, Suh DH, Kim JW. Major clinical research advances in gynecologic cancer in 2020. J Gynecol Oncol. 2021;32:e53.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||