AbstractPurposeWe aimed to investigate the feasibility of four criteria on oligometastasis (OM) concerning clear survival benefits of local therapy (LT) during tyrosine kinase inhibitor (TKI) treatment in non–small cell lung cancer (NSCLC).
Materials and MethodsThis single-center, retrospective study included patients with advanced NSCLC who received LT because of OM during TKI treatment at Asan Medical Center from January 2011 to December 2020. At the application of LT OM was classified according to four criteria: TNM, European Organization for Research and Treatment of Cancer Lung Cancer Group (EORTC-LCG), National Comprehensive Network (NCCN), and ORGAN. We compared survival outcomes between patients with and without OM.
ResultsThe median overall survival of the 117 patients included in the analysis was 70.8 months (95% confidence interval [CI], 56.6 to 85.1). The patients with OM meeting all four criteria (hazard ratio [HR] with 95% CI of TNM criteria 0.24 with 0.10–0.57; p=0.001, EORTC-LCG criteria 0.34 with 0.17–0.67; p=0.002, NCCN criteria 0.41 with 0.20–0.86; p=0.018 and ORGAN criteria 0.33 with 0.18–0.60; p < 0.001) had significantly longer survival compared with patients who did not after adjusting for confounding factors. Furthermore, increasing the number of extra-thoracic metastatic organs to two or more were independent predictive factors for worse survival outcomes (2 organs: HR, 3.51; 95% CI, 1.01 to 12.14; p=0.048; 3 organs: HR, 4.31; 95% CI, 0.94 to 19.73; p=0.060; 4 organs: HR, 24.47; 95% CI, 5.08 to 117.80; p < 0.001).
IntroductionOligometastatic cancer is an intermediate state of malignant neoplasm with the potential to range from localized to widely disseminated disease states [1]. Most patients with non–small cell lung cancer (NSCLC) are also diagnosed with metastatic disease [2]. Furthermore, oligometastasis (OM) can develop as a result of acquired resistance during tyrosine kinase inhibitor (TKI) treatment in patients with metastatic NSCLC [3]. This pattern of progression was observed in 38.0% of patients with advanced NSCLC who received epidermal growth factor receptor (EGFR)-TKIs [4]. For these patients, local therapy (LT) with EGFR TKI maintenance is a reasonable option with significantly improved clinical benefit [5].
The definition of oligometastatic NSCLC has varied widely across several studies [6]. Traditionally, OM was broadly defined as a state of limited metastatic tumor burden, with one or several sites of metastasis [7]. However, these criteria have now increased to span the number of metastatic lesions, organs involved, or specific sites [8]. The National Comprehensive Network (NCCN) treatment guidelines recommend LT for patients with limited metastatic disease, ranging from three to five metastases [9]. On the other hand, the European Organization for Research and Treatment of Cancer Lung Cancer Group (EORTC-LCG) has defined OM as metastatic status at one to five sites and up to three organs [10]. The lack of universal criteria for OM has posed significant challenges in determining treatment paradigms for specific patient groups [11]. We therefore analyzed the feasibility of the current four OM criteria in assessing the clear survival benefit by LT during TKI treatment.
Materials and Methods1. PatientsA retrospective study was conducted on patients with advanced NSCLC treated by LT for oligometastatic lesions during TKI therapy between January 2011 and December 2020 at Asan Medical Center. Patients who met the following criteria were enrolled: (1) ≥ 18 years old with pathologically confirmed NSCLC; (2) stage III or IV disease according to the 8th edition of the American Joint Committee on Cancer Staging system; (3) receiving targeted TKI therapy (first-line in untreated patients or subsequent treatment for progressive disease); (4) having a pattern of OM during TKI treatment; (5) receiving LT for oligometastatic lesions with maintenance TKI therapy. Patients underwent routine surveillance imaging for the treatment response every 6–8 weeks after the initiation of therapy per the general practice guidelines of our institution. A bone or positron emission tomography scan was performed when bone metastasis or systemic progression was suspected.
2. Criteria of OMOligometastatic disease was defined as the development of new lesions or newly progressive lesions alongside a majority of well-controlled lesions in patients receiving TKI therapy for advanced lung cancer. During LT, patients were divided into the OM group and the non-OM (NOM) group according to the four following criteria (S1 Table). First, the TNM criteria defined OM as the metastatic status of M1a and M1b according to the 8th edition of TNM staging [12]. Second, the EORTC-LCG criteria defined OM as the presence of ≤ 5 lesions in 1–3 organs [13]. Third, the NCCN criteria considered up to three metastatic lesions as OM [9]. Finally, we provided the new criteria by specifying a single number of extra-thoracic organs involved to be included in the oligometastatic state regardless of the number of lesions [14]. The schema of this study is illustrated in S2 Fig.
3. Study outcomes and variablesThe primary outcome of this study was to compare overall survival (OS) between the OM group and the NOM group under the four OM criteria. The secondary outcomes were progression-free survival (PFS) from the date of LT to systemic progression (PFS1) and from TKI initiation to systemic progression (PFS2). We additionally analyzed the association between the number of extra-thoracic metastatic lesions or involved organs and survival outcomes.
Baseline characteristics were obtained from electronic patient records, including age, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, tumor pathology, TNM stage (8th edition), mutational status, type of TKI, the number of sites, and oligometastatic disease at initiation of LT, type of LT, sites of LT, and subsequent treatment. The LT type included surgery, radiotherapy, stereotactic radiosurgery (SRS), or both. In this study, SRS was defined as radiation therapy targeting high doses of radiation to brain lesions, with minimal impact on the surrounding tissue using 3D imaging. Other forms of radiation treatment—such as palliative radiotherapy to bone lesions or radiotherapy to the whole brain—were included in the broader radiotherapy category.
EGFR mutation was detected by polymerase chain reaction from paraffin-embedded tumor samples. Fluorescence in situ hybridization was used to identify anaplastic lymphoma receptor tyrosine kinase (ALK) rearrangement, and treatment response was evaluated according to the Response Evaluation Criteria in Solid Tumors ver. 1.1.
4. Statistical analysisData were presented as numbers with percentages for categorical variables and means plus standard deviations or median with interquartile range for continuous variables. The chi-square test or Fisher exact test was used to compare categorical variables, while the Student’s t test or Mann-Whitney U test was used to compare continuous variables with normal or non-normal distribution. The Kaplan-Meier method was used to estimate survival curves for time-to-event analysis, whereas the log-rank test was used to test the significance of the differences. PFS was defined as the duration from treatment initiation until the date of progression, the development of a new lesion, death, or last follow-up. OS was calculated from the date of commencement of TKI treatment until the date of death from any cause. Univariable and multivariable Cox proportional-hazards regression models were used to identify risk factors associated with survival outcomes. A final model was constructed using a stepwise method with backward selection. p-values < 0.15 in the univariate analysis were set for the entry of variables. The proportional hazards assumption was assessed through inspection of Schoenfeld residuals. Two-sided p-values < 0.05 were considered to indicate significance. All analyses were performed using SPSS software ver. 26.0 (IBM Corp., Armonk, NY).
Results1. Baseline characteristicsOverall, 117 patients with mutant NSCLC receiving LT were included in the present study. The baseline characteristics are presented in Table 1. The median age of all patients was 59.3 years, and the proportion of female and never smokers was 59.0% and 62.4%, respectively. Most ECOG performance statuses (98.9%) were between 0 and 2, while only one patient had a mixed pathology with squamous cell carcinoma and adenocarcinoma. Among all patients, 72 (61.5%) and 41 (35.0%) cases had nodal and pleural metastases, respectively. Approximately 66% of cases were classified as M1c stage. A total of 88 patients (75.2%) received TKI treatment as first-line chemotherapy. Among the 116 patients with identified mutational status, 47 (40.5%) had exon 19 deletion, 34 (29.3%) had exon 21 L858R, 10 (8.6%) had exon 20 T790M, and 20 (17.2%) had ALK arrangement. The most common type of TKI was EGFR TKI (82.9%), whereas only 20 patients (17.1%) received ALK TKI. The proportion of patients with partial response at the initial evaluation was 81.0%. Seven patients (6.0%) underwent the LT operation, 68 patients (58.1%) received radiotherapy, and 44 patients (37.6%) were treated with SRS (Table 2). Subsequent chemotherapy was received by 65 patients (55.6%) after the cessation of TKI. The baseline characteristics between OM and NOM groups in line with the four definitions are fully described in Tables 3 and 4.
2. Clinical outcomesFor the entire cohort, the median OS, PFS1, and PFS2 were 70.8 months (95% confidence interval [CI], 56.6 to 85.1), 10.3 months (95% CI, 5.5 to 15.1), and 30.9 months (95% CI, 20.6 to 41.1), respectively (Fig. 1). The outcomes of the 117 patients included in this analysis were much better than those of the historic controls (median, 27.9 to 38.6 months of OS and median, 11.0 to 17.7 months of PFS2) [15,16]. After classifying the OM group according to four criteria, the clinical outcomes were compared between the OM group and NOM groups. Patients in the OM group under the TNM criteria (median, undefined vs. 63.3 months; p < 0.001), EORTC-LCG criteria (median, 111.0 vs. 62.6 months; p=0.001), NCCN criteria (median, 111.0 vs. 63.3 months; p=0.013), and ORGAN criteria (median, 84.2 vs. 37.5 months; p < 0.001) had significantly longer OS than patients in the NOM group (Fig. 2A). Additionally, the differences in PFS were statistically significant between the OM and NOM groups for all four criteria. Patients in the OM group according to the TNM criteria (median, 44.7 vs. 9.2 months; p < 0.001), EORTC-LCG criteria (median, 18.3 vs. 9.2 months; p=0.008), NCCN criteria (median, 23.7 vs. 9.5 months; p=0.011), and ORGAN criteria (median, 18.7 vs. 8.9 months; p < 0.001) had significantly greater PFS1 than those in the NOM group (Fig. 2B). PFS2 in the OM group was also significantly improved when defined by the TNM criteria (median, 66.0 vs. 27.1 months; p < 0.001), EORTC-LCG criteria (median, 51.8 vs. 27.6 months; p=0.001), NCCN criteria (median, 54.1 vs. 27.6 months; p=0.005), and ORGAN criteria (median, 44.8 vs. 21.4 months; p < 0.001) (Fig. 2C).
3. Outcomes after adjustment for co-variablesAfter adjustment for potential confounders through the Cox proportional hazards analysis, the OM group under the TNM criteria (all-cause death: adjusted hazard ratio [HR], 0.24; 95% CI, 0.10 to 0.57; p=0.001; PFS1: adjusted HR, 0.33; 95% CI, 0.18 to 0.61; p < 0.001; PFS2: adjusted HR, 0.29; 95% CI, 0.16 to 0.52; p < 0.001), NCCN criteria (all-cause death: adjusted HR, 0.42; 95% CI, 0.20 to 0.86; p=0.018; PFS1: adjusted HR, 0.54; 95% CI, 0.32 to 0.89; p=0.016; PFS2: adjusted HR, 0.52; 95% CI, 0.31 to 0.89; p=0.016), and ORGAN criteria (all-cause death: adjusted HR, 0.33; 95% CI, 0.18 to 0.60; p < 0.001; PFS1: adjusted HR, 0.38; 95% CI, 0.24 to 0.62; p < 0.001; PFS2: adjusted HR, 0.32; 95% CI, 0.20 to 0.51; p < 0.001) remained significantly associated with better outcomes compared with the NOM group (Fig. 3). Contrary to the results of other criteria, the OM group under the EORTC-LCG criteria was not statistically associated with improved PFS1 (adjusted HR, 0.68; 95% CI, 0.41 to 1.11; p=0.119).
4. Association between the number of extra-thoracic metastatic lesions or involved organs and the clinical outcomesNext, we examined the associations between the number of extra-thoracic metastatic lesions or involved organs with PFS2 and OS. In the adjusted Cox proportional hazards analysis, PFS2 was significantly lower in patients who had at least two extra-thoracic metastases (1 organ: adjusted HR 1.38; 95% CI, 0.61 to 3.13; p=0.446; 2 organs: adjusted HR, 4.32; 95% CI, 1.80 to 10.35; p=0.001; 3 organs: adjusted HR, 2.98; 95% CI, 1.08 to 8.26; p=0.035; 4 organs: adjusted HR, 15.20; 95% CI, 4.11 to 56.24; p < 0.001) (Table 5). Consistently, multivariate analysis on OS revealed the greater number of extra-thoracic metastatic organs of two or more was an independent predictive factor for lower OS; however, there was no statistical association between three extra-thoracic metastatic organs and OS (1 organ: adjusted HR, 1.82; 95% CI, 0.54 to 6.10; p=0.334; 2 organs: adjusted HR, 3.51; 95% CI, 1.01 to 12.14; p=0.048; 3 organs: adjusted HR, 4.31; 95% CI, 0.94 to 19.73; p=0.060; 4 organs: adjusted HR, 24.47; 95% CI, 5.08 to 117.80; p < 0.001). However, there were no significant associations between the number of extra-thoracic metastatic lesions and either PFS2 or OS, even after adjusting for confounders, except in the case of five or more extra-thoracic metastatic lesions (≥ 5 lesions: adjusted HR 3.92 [1.74–8.82], p=0.001 for PFS2 and 4.86 [1.42–16.56], p=0.012 for OS).
DiscussionThis retrospective, single-center study was conducted to investigate the efficacy of four criteria on assessing OM in LT-treated NSCLC during TKI therapy. Among patients with advanced NSCLC who received TKI treatment, patients meeting all four criteria for OM showed significantly greater prognostic benefits from LT than those with NOM even after adjusting for confounders. Conventionally, oligometastatic cancer has been defined by the number of metastatic lesions. However, this analysis revealed that clinical outcomes were significantly associated with the number of extra-thoracic metastatic organs involved but not the number of extra-thoracic metastatic lesions. Thus, to improve clinical prognosis, physicians should consider the number of extra-thoracic metastatic organs involved when evaluating patients with OM eligible for LT during TKI treatment.
The rationale for LT in NSCLC patients with OM during TKI treatment is based on the concept of oligometastatic cancer as an intermediate state extending from local to systemic disease [1]. The metastatic cascades occur when one metastatic site evolves the capacity to seed other metastases [17]. Although the trajectory of metastases evolution varies according to intratumoral heterogeneity, disease histology, and the nature of the patient, one or multiple progenitor metastases could yield a cascade of numerous emerging metastases given enough time [18]. Establishing metastasis requires the completion of several steps [19]. In particular, the potential for metastatic colonization, which is the final step in metastasis, is dependent on the specific target organ, noted as the seed and soil hypothesis [20]. A therapeutic window may therefore exist for ablation of these progenitors if detected early enough in certain patients, potentially halting the emergence of polymetastatic disease [14]. Thus, patients who developed OM during TKI therapy could benefit from LT with continual TKI administration. A previous study on LTs, including surgery, radiation, or radiofrequency ablation for EGFR-mutant NSCLC during TKI therapy, found median times to progression after LT of 10 months, which was similar to our median PFS1 (10.3 months). The median OS from LT was 41 months, which was longer than the corresponding finding in a previous study reporting on EGFR TKI [21,22]. Another study on ALK-mutated NSCLC that progressed in fewer than five lesions on crizotinib revealed improved clinical outcomes in patients with radiotherapy compared with those without LT [23]. Additionally, a retrospective study conducted on oncogene-addicted NSCLC with limited systemic disease reported that it might be reasonable to consider LT as the site of progression and continuation of the TKI [24]. Survival benefits obtained from LT in our study were in line with the results of previous research, demonstrating better prognosis of NSCLC on TKI therapy than historical data [25]. These data suggest that the use of LT during TKI therapy can improve the survival outcomes of patients with NSCLC.
Our study showed that patients with OM derived better survival benefits from LT than those with NOM, emphasizing the importance of precise criteria for oligometastatic cancer before LT. Our observations implied that the two or more extra-thoracic metastatic organs involved were significantly associated with prognosis. Thus, it is important to select patients with OM using efficacious criteria for the best clinical outcomes. Progressive lesions during TKI therapy developed slowly in all OM groups according to the four criteria compared with the NOM group. However, our study found no statistical association between PFS after LT and OM defined by EORTC-LCG criteria in the multivariate analysis. The definitions of EORTC-LCG were mainly based on the number of metastatic lesions [13]. Our data indicate that the number of extra-thoracic metastatic lesions have no significant association with clinical outcomes; therefore, the EORTC-LCG criteria may be limited in accurately distinguishing patients with OM who may benefit from LT. Similar to the EORTC-LCG criteria, the NCCN criteria classify the status of OM by the number of metastatic lesions. However, because we set the NCCN criteria to three lesions or less, only 11.8% of the patients with OM had two or more extra-thoracic metastatic organs according to the NCCN criteria. Therefore, the number of extra-thoracic metastatic organs involved could be pivotal for selecting oligometastatic disease with prognostic advantage.
Since the TNM criteria consisted of M1a, which included intrapulmonary metastasis, and M1b, which included single extra-thoracic metastasis, the maximum number of extra-thoracic metastatic organs involved was one or less in the TNM criteria, similar to the ORGAN criteria. The OM groups using TNM criteria had the longest PFS and OS than other criteria, while the number of patients with OM was the smallest among the four OM groups. Regardless, the number of enrolled patients in the OM group under the ORGAN criteria was approximately twice as large as those per the TNM criteria. Although the OM group under the ORGAN criteria had inferior results to those defined by the TNM criteria, they also showed better outcomes than the historic data [15,16,22]. Therefore, certain groups that may benefit from LT could be missed if only the TNM criteria are used.
Along with attempts to find clinical factors, efforts to identify biologic characteristics predictive of oligometastatic versus polymetastatic cancer have continued in order to improve the criteria for oligometastatic patient selection. Specifically, numerous studies have focused on generating robust signature biomarkers for patient selection. One key study evaluating resection specimens from pulmonary metastasectomy found an mRNA expression signature predictive of oligometastatic progression and survival [26]. Interestingly, ectopic expression of OM-associated microRNAs suppresses proliferation to limit the number of meta-stases in vivo studies [27]. Moreover, intratumoral heterogeneity is associated with disease progression [28]. The liquid biopsy with the noninvasiveness and ease of repeated sampling has recently emerged as a valuable platform for identifying OM biomarkers. Liquid biopsies can successfully monitor clonal heterogeneity of breast cancer and detect aberrant methylation changes of specific tumor genomic loci [29]. This potentially revolutionary tool for molecular testing may eventually guide optimal patient selection in the future.
There are several limitations to our study. First, this was a retrospective study in a single institution, which may have resulted in selection bias. The median survival of our cohort was longer than those of historical data, which may be because only patients who responded well to TKI were included in our study. Second, this study did not use a predefined protocol to evaluate the response to treatment and subsequent therapy, owing to the retrospective nature of the study. The actual practice after the assessment of response was at the discretion of the treating physician. This could have affected the comparison of the true benefit between the OM and NOM groups. Third, the type of TKIs was somewhat heterogeneous. Although these were considered the standard-of-care treatments at the time of the study, the results needed to be interpreted with caution. Finally, the sample size was not large enough to detect a statistical difference in some subgroups. In our study, only six patients received an operation as LT. Although patients treated by operation had significantly shorter PFS than those with other LTs, we could not further examine the different effects according to the type of LT due to the limited sample size.
In conclusion, this study represents an effort to investigate the feasibility of the current four criteria defining OM with respect to clinical benefit when patients with advanced NSCLC during TKI treatment received LT. Our results found that patients with OM defined by all four criteria showed prognostic benefits from LT during TKI therapy. In addition, the increased number of extra-thoracic metastatic organs to two or more was an independent predictive factor for worse outcomes. This study may provide a rationale for considering the number of extra-thoracic metastatic organs involved in the application of LT to oligometastatic cancer. Further multicenter, prospective studies are needed to confirm these findings.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Asan Medical Center Institutional Review Board (IRB No. 2021-0034). The requirement for informed consent was waived by the Asan Medical Center Institutional Review Board because of the retrospective nature of the study. All work was conducted according to the Guidelines for Good Clinical Practice and the 1964 Declaration of Helsinki. Author Contributions Conceived and designed the analysis: Jang YJ, Hyun DG, Lee JC. Collected the data: Hyun DG, Jang YJ. Contributed data or analysis tools: Ji W, Choi CM, Lee DH, Kim SW. Performed the analysis: Jang YJ, Hyun DG, Yoon S, Lee JC. Wrote the paper: Jang YJ, Hyun DG, Ji W, Lee DH, Kim SW, Lee JC. Interpretation and review and comment: Choi CM, Yoon S. AcknowledgmentsThis study was supported by the University of Ulsan College of Medicine. Editorial support in the form of writing assistance, collation of the authors’ comments, and grammatical editing was provided by the Scientific Publications Team of the Asan Medical Center.
Fig. 1Kaplan-Meier curves of clinical outcomes in all non–small cell lung cancer patients with oligometastasis after the commencement of tyrosine kinase inhibitor (TKI). (A) Overall survival (OS) from the commencement of TKI. (B) Progression-free survival (PFS) from local therapy to systemic progression. (C) PFS from the commencement of TKI to systemic progression. CI, confidence interval. 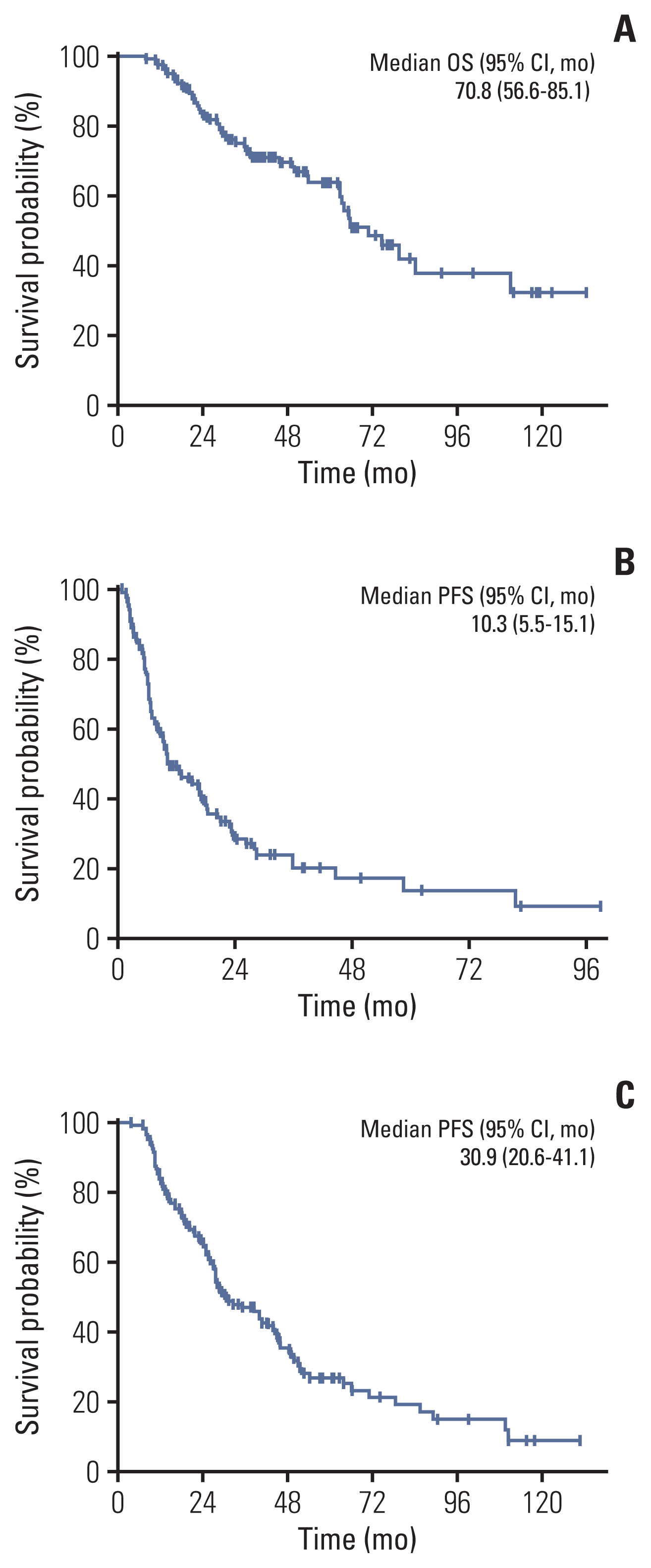
Fig. 2Kaplan-Meier curves of clinical outcomes for subgroup analyses according to the criterion of oligometastasis. (A) Overall survival from the commencement of tyrosine kinase inhibitor (TKI). (B) Progression-free survival from local therapy to systemic progression. (C) Progression-free survival from the commencement of TKI to systemic progression. EORTC-LCG, The European Organization of Research and Treatment of Cancer Lung Cancer Group; NCCN, National Comprehensive Cancer Network. 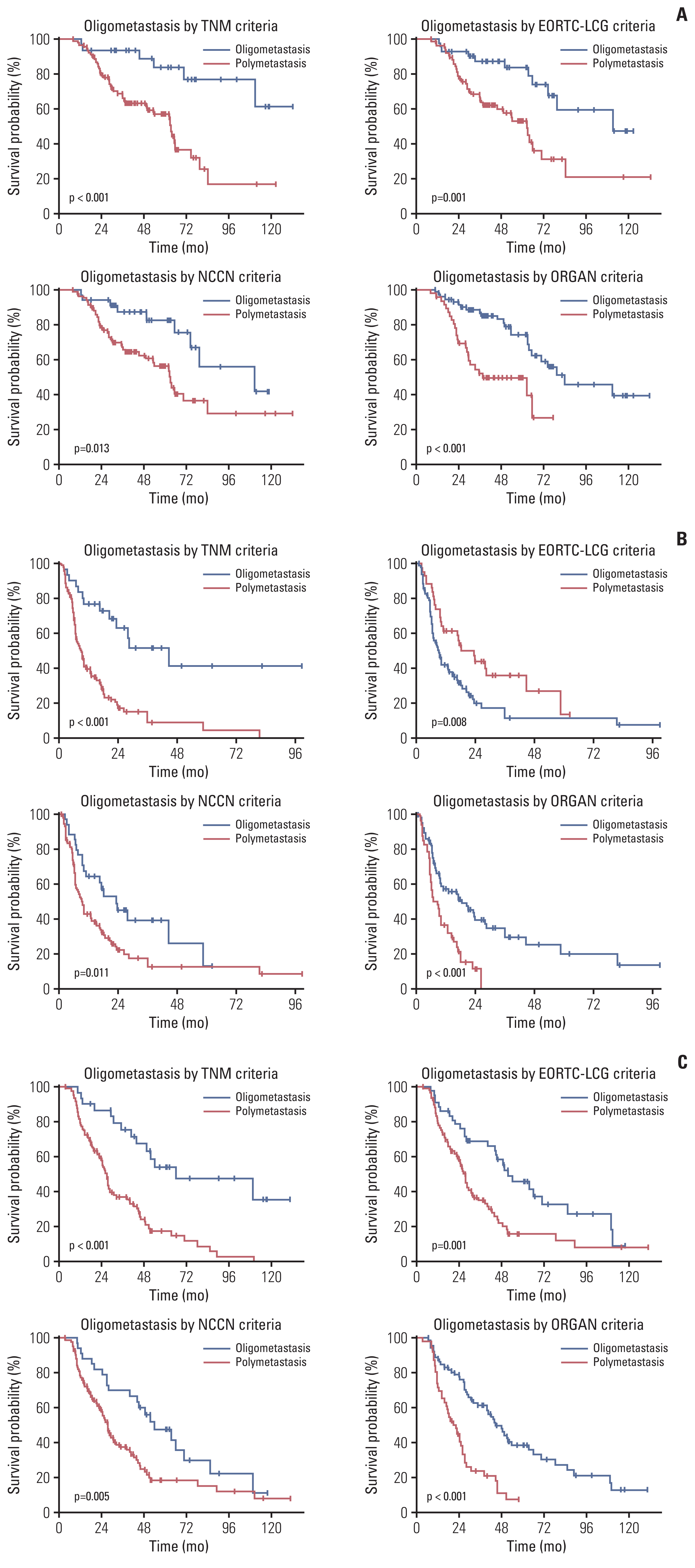
Fig. 3Risk-adjusted hazard ratios (HRs) associated with clinical outcomes according to the criterion of oligometastasis (OM). Shown were HRs, with 95% confidence interval (CI), which were performed with the use of a Cox proportional hazards model. An HR of less than 1.00 indicates a lower risk of clinical outcomes with OM group than non-OM group. Co-variable selection was based on statistical significance. Progression-free survival (PFS) 1 was defined as PFS from local therapy to systemic progression, and PFS2 as PFS from the commencement of tyrosine kinase inhibitor to systemic progression. EORTC-LCG, The European Organization of Research and Treatment of Cancer Lung Cancer Group; NCCN, National Comprehensive Cancer Network. 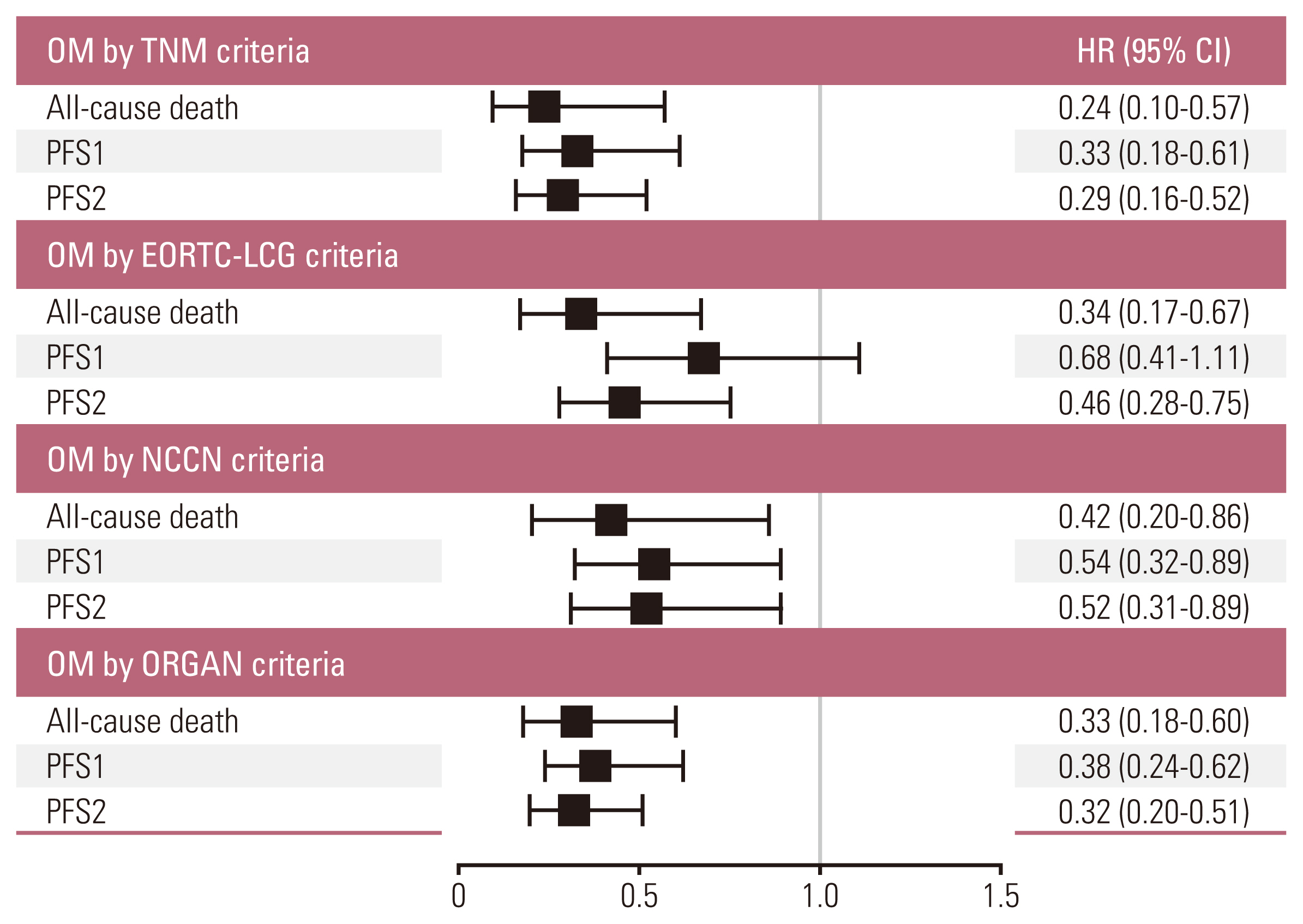
Table 1Baseline characteristics of all patients at TKI initiation
Table 2Profile of local therapy and subsequent treatments
Table 3Baseline characteristics of all patients at the commencement of TKI according to the criterion of oligometastasis
Values are presented as mean±SD or number (%). ALK, anaplastic lymphoma receptor tyrosine kinase; ECOG PS, European Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; EORTC-LCG, The European Organization of Research and Treatment of Cancer Lung Cancer Group; NCCN, National Comprehensive Cancer Network; NOM, non-oligometastasis; OM, oligometastasis; SD, standard deviation; TKI, tyrosine kinase inhibitor. Table 4Profile of local therapy and subsequent treatments according to the criteria for oligometastasis
Values are presented as number (%). ALK, anaplastic lymphoma receptor tyrosine kinase; EGFR, epidermal growth factor receptor; EORTC-LCG, The European Organization of Research and Treatment of Cancer Lung Cancer Group; NCCN, National Comprehensive Cancer Network; NOM, non-oligometastasis; OM, oligometastasis; TKI, tyrosine kinase inhibitor. Table 5Cox proportional-hazards analysis of the number of metastatic lesions or involved organs and clinical outcomes References2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
3. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–81.
4. Li XY, Zhu XR, Zhang CC, Yu W, Zhang B, Shen TL, et al. Analysis of progression patterns and failure sites of patients with metastatic lung adenocarcinoma with EGFR mutations receiving first-line treatment of tyrosine kinase inhibitors. Clin Lung Cancer. 2020;21:534–44.
5. Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13:1383–92.
6. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15:346–55.
8. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8.
9. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19:254–66.
10. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28.
11. Blumenthaler AN, Antonoff MB. Classifying oigometastatic non-small cell lung cancer. Cancers (Basel). 2021;13:4822.
12. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203.
13. Dingemans AC, Hendriks LE, Berghmans T, Levy A, Hasan B, Faivre-Finn C, et al. Definition of synchronous oligometastatic non-small cell lung cancer: a consensus report. J Thorac Oncol. 2019;14:2109–19.
14. Correa RJ, Salama JK, Milano MT, Palma DA. Stereotactic body radiotherapy for oligometastasis: opportunities for biology to guide clinical management. Cancer J. 2016;22:247–56.
15. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
16. Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28:270–7.
17. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92.
18. Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol. 2015;12:258–72.
19. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
20. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8.
21. Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–51.
22. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66.
23. Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–8.
24. Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–14.
25. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–29.
26. Lussier YA, Khodarev NN, Regan K, Corbin K, Li H, Ganai S, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7:e50141.
27. Uppal A, Wightman SC, Mallon S, Oshima G, Pitroda SP, Zhang Q, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget. 2015;6:3540–52.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||