AbstractNext-generation sequencing (NGS) is becoming essential in the fields of precision oncology. With implementation of NGS in daily clinic, the needs for continued education, facilitated interpretation of NGS results and optimal treatment delivery based on NGS results have been addressed. Molecular tumor board (MTB) is multidisciplinary approach to keep pace with the growing knowledge of complex molecular alterations in patients with advanced solid cancer. Although guidelines for NGS use and MTB have been developed in western countries, there is limitation for reflection of Korea’s public health environment and daily clinical practice. These recommendations provide a critical guidance from NGS panel testing to final treatment decision based on MTB discussion.
IntroductionNext-generation sequencing (NGS) and its increased cost-efficiency have allowed for the rapid panel testing of hundreds of genes [1–4] and the selection of tumor mutations and targeted anticancer drugs [5]. Therefore, the clinical application of NGS in the fields of Precision Medicine and Precision Oncology is increasing [1]. In Korea, the reimbursed healthcare system began to cover NGS panel testing in 2017, which has led to an increase from 4,000 tests in 2017 to 10,000 in 2019 [6]. The process for clinical use of NGS includes (1) obtaining the tumor tissue from a patient with cancer, (2) extracting nucleic acids (DNA and/or RNA) for sequence analysis, (3) confirming the tumor genomic alterations and formulating a report accordingly, and (4) deciding to proceed with evidence-based, personalized treatment or a clinical trial through the molecular tumor board (MTB).
In Korea, the Korean Society of Medical Oncology (KSMO) and Korean Cancer Study Group (KCSG) jointly founded Korean Precision Medicine Networking Group (KPMNG) in 2019 and have been working to improve outcomes for patients with cancer by applying Precision Medicine to clinical fields, in collaboration with Korean Society of Pathologists. Although general guidelines have been developed in some western countries to interpret NGS results and the oncology board (MTB), their application is limited owing to inaccurate reflection of Korea’s public health specificities and daily clinical practice.
Thus, expert agreement is needed to suggest considerations for clinicians in the prescription of NGS and subsequent analyses of patients with advanced solid cancer, including genomic data analysis (NGS) and tumor board (MTB) in Korea. The purpose of these recommendations is to (1) provide an opportunity to share relevant clinical insights and experience among experts through the treatment guidelines development process, (2) suggest a systematic process to oncology experts when conducting NGS tests, interpreting results, and making relevant therapeutic decisions, and (3) help determine the optimal treatment strategies through multidisciplinary approaches by suggesting the effective operation of the MTB.
MethodsThe formulators of these recommendations consisted of a Steering Committee and a Writing Committee throughout November 2020. The Steering Committee comprised oncology specialists convened by the KSMO and the KCSG, pathologists recommended by the Korean Society of Pathologists, and bioinformatician expert recommended by KPMNG. The Writing Committee was organized by members representing a total of eight disease divisions recommended by the KCSG. The Steering Committee and the Writing Committee selected key questions through two workshops and two meetings from 2020 to 2021. The final six key questions were developed by conducting two surveys of the Steering and Writing Committees. These questions included the performance and interpretation of the NGS testing regardless of cancer type, the process of performing the MTB and proposing personalized treatment after receiving reports, the timing of the NGS testing according to cancer type and stage, and the list of actionable genes to be tested for each cancer type. The Steering Committee wrote general issues concerning each question, and disease-specific questions were addressed by the Writing Committee. The recommendations made by the Committees were finalized by confirming reflection of the opinions of the medical oncologists at the annual symposium of the KSMO in May 2021.
The Committees evaluated the current clinical landscape in Korea according to international guidelines and research to address each question. This paper will cover key questions, while more specific questions will be announced separately (optimal timing of NGS and actionable genes according to cancer type). The developed recommendations were peer-reviewed and revised through mutual verification.
Key Questions and RecommendationsThe key questions are presented in Table 1.
1. What considerations should be made when obtaining samples for NGS?Target areas of DNA extraction are the most important consideration for pathologists before NGS DNA preparation, and enrichment of tumor cells is vital when selecting the region of interest. Extracting DNA from locations below the standard for tumor cell fraction can lead to false negatives. If highly probable mutations are not found during the NGS, such as APC mutation in colorectal cancer, the tumor cell fraction at the DNA extraction point should be suspected of being too low. Furthermore, mixed normal cells with diploid genomes may dilute the amplification signal; therefore, thorough evaluation of tumor cell fraction and correction of the measured copy number are vital. Sample selection should therefore be made under close communication between clinicians and pathologists.
When comparing the primary and recurring/metastatic sites in each specimen, it is important to consider that primary tumors and metastatic tumors before treatment with systemic chemotherapy share a large proportion of key tumor-related genetic alterations [7–9]. Nevertheless, recurred or progressed tumors can be accompanied by genomic evolution. Although the majority of the additional genomic alterations are likely to be passenger [10], there can be therapeutically relevant alterations that mediate drug-resistance or predict patient prognosis. Therefore, re-biopsies at recently obtained relapse sites are more likely to reflect the solid tumor’s genetic features at the time of NGS. However, if clinical circumstances prevent the obtainment of recurring/metastatic tissue, primary tumors may be used.
Tumor purity, described as the proportion of tumor cells, is an important factor influencing the reliability of the examination because low tumor purity can potentially lead to false-negative outcomes. Suitable NGS samples should have the highest tumor purity and cellularity among biopsy and surgical samples, and surgical samples are generally preferred. However, the quantity of viable tumor cells in the surgical sample should be considered during selection, which may be too low if neoadjuvant therapy was conducted. The minimum requirement for tumor purity varies according to the NGS analysis method and technical sensitivity; however, a higher content of nucleic acids and tumor cells with higher yields reduce the chance of false negativity. Regardless, some samples such as sclerotic or cystic tumors tend to have low cellularity or may contain necrotic regions that can affect nucleic acid yield. All factors should therefore be reviewed if a false-negative result occurs. NGS requires a minimum quantity of 100 ng of DNA for hybrid capture and 10 ng for amplicon. Notably, hybrid capture requires at least 1 mm2 of tumor tissue under exhaustive conditions, necessitating more than 2 mm (diameter) endoscopic biopsy tissues of three sections and at least two unstained slides of surgical tissue [11]. However, the minimum amount is dependent on the quality of the samples, so securing unstained slides at 1.5× the minimum requirement is recommended (i.e., 10 biopsy tissue slides, five surgical tissue slides). These criteria are reference only, establishing an optimized protocol tailored to the institution’s experience is advised.
Tissue storage and fixation is crucial to achieve high-quality samples suitable for NGS. Delayed or insufficient fixation in surgical samples, in particular, may lead to DNA degradation [12]; sufficient fixation can be achieved by placing the sample in buffered formalin suitable for the volume of the tissue within 1 hour of acquisition. Additionally, high-quality preoperative biopsy samples may be used if surgical tissue has been improperly fixated. The possibility of cytosine deamination (C:G > T:A mutations) causing background noise should be considered if tissues are stored for long periods under formalin-fixed paraffin-embedded conditions [13,14].
Liquid biopsies have been introduced into clinical practice for non-invasive genome analysis, treatment response monitoring, identification of drug-resistant mechanisms, early detection of recurrence, and overcoming intra-tumoral heterogenicity by supplementing the limitations of NGS examinations using existing tumor tissues [15].
2. How can the classification level of genes applicable to Korea be determined?Genetic aberrations detected by NGS are classified according to clinical usefulness and then applied to treatment. Although a scoring system for clinical usefulness has been established in several countries, the need for Korea-specific criteria has been highlighted [16,17]. Therefore, this current statement proposes the KPMNG scale of Clinical Actionability of molecular Targets (K-CAT), referring to the existing tier system and considering its applicability to Korean patients (Tables 2 and 3). Level 1 of this scale refers to recognition of a therapeutic agent as a standard treatment for a specific target gene alteration, approved by the Ministry of Food and Drug Safety (MFDS) in Korea or corresponding international regulatory agencies (U.S. Food and Drug Administration [FDA], European Medicines Agency [EMA], etc.) or supported by prospective, randomized, phase III trials showing the benefit of survival endpoints. Treatment drugs targeted for genes corresponding to Level 1 should be subject to reimbursement application. Level 2 denotes a target gene with clinical benefits in prospective phase 1 or 2 studies. A level 2 actionable gene needs to be considered subject to MFDS approval and potential off-label drug use after approval from the Health Insurance Review and Assessment Service (HIRA). Level 3 denotes a gene with potential for clinical benefit, confirmed by retrospective outcomes of specific therapeutic agents in patients with corresponding genes in the same or different indications. Oncologists may consider clinical trial participation or applying for the Expanded Access Program for level 3 results. Level 4 is defined as securing evidence for a therapeutic drug at the laboratory level for the corresponding genes with limited immediate application to clinical treatment decisions. Level G is classified as indication of suspected actionable germline variant, for which following germline tests are recommended. Level R indicates a drug-resistant gene for a specific targeted anticancer drug. This classification of resistant genes can also be used as conditions for insurance coverage and approval for anticancer drugs.
K-CAT aimed to present methods and grounds for NGS results for application to treatment decisions reflecting the domestic situation in Korea. Gene classification depends on whether studies were prospective, were approved by the MFDS, the magnitude of survival benefits, data on other indications and the preclinical data. Table 3 presents the criteria reflected in each scale. K-CAT list of genes for each cancer type will be addressed separately in the next series of KPMNG guidelines.
3. What are the considerations for interpreting the results of the NGS?The testing environment for the NGS differs by each institution in terms of sequencers (Illumina NextSeqDX or Thermofisher ion S5), chemistry used in panels (amplicon or hybrid capture method), test range (number of genes included in the panel and kind of variants to be detected), and detection criteria of variants. Regarding that detection results may vary in the boundary conditions, it requires further caution when comparing and interpreting the results provided by different institutions.
From sample collection to the analysis step, the final results can be affected by diverse variables [18]. To be specific, the quantity and quality of tumor samples as well as the NGS library preparation, sequence alignment, and analysis software can cause the variables, which can lead to various false positives in the results. Although such false positives are filtered out as much as possible throughout both analysis and manual curation steps, some indecisive variants may be included in the final reports as “variants of uncertain/unknown significance (VUS).” Therefore, VUS should be interpreted with further caution.
Above all, since most NGS tests do not use matched normal samples, germline and somatic variants cannot be easily distinguishable, and therefore potential germline variants are possibly included in the results. Hence, if a pathogenic germline variant is suspected, it is recommended to confirm the variant in normal tissues or blood samples via appropriate genetic counseling.
In general, the limit of detection (LOD) to report variant detection is set to a variant allele frequency (VAF) threshold of 3%–5% [18]. However, in cases of tumor purity is low, the final results may include significant variants (such as hotspot or oncogenic) below the LOD (1%–2%). The VAF of the detected somatic variants should be considered based on the tumor purity level. In particular, the purity level and VAF have similar distributions. If VAF is approximately half of the tumor purity level, the variant is considered a heterozygous loss variant. Variants with different levels of the VAF other than the aforementioned cases can be interpreted as variants that emerged from subclones.
Fusions are fundamental markers for cancer diagnosis, patient prognosis, and targeted therapy. Most fusion breakpoints occur between intronic regions. For accurate detection of fusions, gene panels should be designed with optimization to cover either whole regions of a gene or specific exonic/intronic regions where fusions frequently occur. However, intronic regions are hardly covered by the sequencing due to repeated sequences. In this respect, no fusion is detected if breakpoints are located in the uncovered regions. To overcome these limitations, the use of RNA panels has been gradually expanded recently [19].
In most cases of copy number variations (CNVs), only variants with amplifications or homozygous deletions (0 copy) are reported according to the reliability of the results, and variants with heterozygous deletions (1 copy) are usually not reported due to possible experimental errors. Additionally, thresholds for genes that are frequently amplified (ERBB2 and MET) and those that are not frequently amplified are flexibly applied, respectively. In the “frequently amplified” genes, it can be suspected to have an amplification even though the copy number is low. Thus, in these cases, confirmation by comparing results obtained from alternative methods (such as fluorescent in situ hybridization) is highly recommended. Besides, CNVs are affected by the number of target probes and amplicons, theoretically. Thus, the copy numbers estimated by using single probes (usually targeting the hotspot regions of a gene) are not accurate as of the average estimation from probes that covers the whole exonic regions.
Although there is no standardized numerical definition for the number of collective tumor mutations per 1 Mb, tumor mutational burden (TMB) has arisen as new biomarker for immune checkpoint inhibitors [20–22]. Regarding that the types of variants included in the TMB definition and calculation are varied as “missense variants only,” “including nonsense and frameshift variants,” or else, it is necessary to check the definition of TMB applied in the panel test. Furthermore, since the thresholds to classify as “TMB-high” are varied from 7.4 mutations per Mb (mut/Mb) to 20 mut/Mb depending on cancer types, it is important to confirm the appropriate LOD for each type of cancer.
Tumors with high microsatellite instability are another key factor for immunotherapy along with TMB. To measure and determine the stability of microsatellite instability, various markers (such as BAT-25, BAT-26, D2S123, D5S346, D17S250, NR21, NR24, and NR27) are used. Since detection criteria are varied by each panel type, the LOD should be thoroughly reviewed [23–26].
4. How should MTB be operated?MTBs are consultative bodies of cancer experts, including medical oncologists, pathologists, and bioinformaticians who gather within the institution/hospital network to share clinical insights and establish optimal treatment strategies based on complex genetic results [27]. Essential personnel for operating MTBs includes medical oncologists, pathologists, and bioinformaticians, and ideally include clinical geneticists, tumor biologists, and clinical research coordinators [28]. However, realistically, many institutions would not be able to operate MTB with such a configuration [29]. Therefore, each hospital should first organize an MTB with available experts, constituting at least a pathologist and an oncologist clinician. If institutional circumstances prevent the MTB from operating at complete configuration, a virtual MTB using an online platform could be an alternative [30,31].
The MTB aims to discuss potential therapeutic options tailored to the results of genetic analyses in patients with advanced solid cancer through a multidisciplinary approach. Essential discussions around the case presented in MTB concern: (1) quality assessment of the NGS, (2) review of clinically significant genetic abnormalities found in the NGS test, and (3) the recommended treatment based on the NGS results [27,28]. Reviewing the use of an appropriate sample and correct test methods comprise the discussions concerning quality within the MTB. Moreover, reviewing appropriate sample selection, cellularity, and mean target depth is important to assess the quality of the NGS. When clinically significant genetic abnormalities arise from the NGS, single nucleotide variants, CNV, and structural variation should be examined. Additionally, when interpreting each genetic aberrations, knowledge databases such as OncoKB (https://www.oncokb.org), CIVic (https://civicdb.org/home), cBioportal (https://www.cbioportal.org/), My Cancer Genome (https://www.mycancergenome.org/), Clinvar (https://www.ncbi.nlm.nih.gov/clinvar/), COSMIC (https://cancer.sanger.ac.uk/cosmic) may be consulted. Other important considerations include addressing findings that may be suspect of microsatellite instability, drug-resistant mutations, and germline mutations. The TMB should also be reviewed to be reflected in the treatment options. The full treatment spectrum should be considered at final treatment selection, including regulatory approved standard treatment, off-label drug use, clinical trials, and the Expanded Access Program. Decision-makers should also refer to crucial factors such as the patient’s previous treatment history, systemic medical condition, and disease course.
The results of the MTB discussion should be recorded in a unified and standardized report, including the patient’s basic information, minutes on the discussion and interpretation of NGS results, and treatment recommendations [27,28]. A virtual MTB report from the Korea Precision Medicine Networking Group Study (KOSMOS) on genomic mutation-based customized drug therapy for patients with advanced solid cancer is presented in Fig. 1.
5. How will NGS results be implemented in optimal precision treatment?An approved standard treatment with a high level of evidence matching NGS-determined genetic variation should be considered preferentially in the MTB. The K-CAT could be used to prioritize recommendations in the event of many identified genetic variations. A medical oncologist should make shared decision making among the proposed treatment considering the patient’s systemic condition, cancer type, disease course, comorbidities, and socioeconomic factors.
If a drug recommended by the MTB based on NGS results is not approved for the particular cancer type, drugs included in the insurance coverage list may be used with the approval of off-label drug use after the review by a multidisciplinary committee of six or more experts, including medical oncologists at each institution, followed by application for HIRA’s approval. Upon prior application, approval for use will be notified after deliberation by the multidisciplinary committee within 60 days of the date of receipt. Patients requiring prompt treatment outside of the pre-approval review period for chemotherapy may apply for post-approval (use before approval) [32].
In addition, if the drug recommended by the MTB is not approved in Korea or does not comply with off-label drug use application conditions, the patient may participate in clinical trials or try the Expanded Access Program. Information regarding clinical trials conducted in Korea may be searched online at the MFDS website (https://nedrug.mfds.go.kr/index), Korea Disease Control and Prevention Agency CRIS (https://cris.nih.go.kr/cris/index/index.do), and KCSG website (https://www.kcsg.org). The Expanded Access Program is a system that allows patients with cancer who can no longer receive standard treatment or participate in clinical trial, to use drugs that are currently under clinical development according to the discretion of an experienced physician after obtaining approval from the MFDS. Since the indications do not have to match those of clinical trials, this process may be considered if medical judgment determines that the benefit of using clinical trial drugs outweighs the risk, with willingness from the pharmaceutical company [33].
To further improve molecular profiling guided therapy, development of better decision-making aids based on the Korean health care situation is needed, as well as a platform for shared decision-making with patients and families [34–36]. Furthermore, it will be essential to build a nationwide clinico-genomic database, integrating genomic data and clinical data including tumor and patient characteristics, prescription data with corresponding treatment outcomes including survival [37–39]. Data privacy protection and appropriate data-sharing strategies should be accompanied to make clinico-genomic database usable to all stakeholders for future research, drug development, practice, drug approval, and reimbursement. Importance of integration and utilization of already established nationwide databases cannot be overemphasized [40].
ConclusionNGS is becoming an essential test for the treatment of patients with advanced solid cancer. This report presents clinical recommendations for the practice of NGS testing in patients with advanced solid cancer in Korea, gene classification ratings appropriate to domestic Korean conditions, considerations for interpreting results, the principles of MTB operation, and practical application of MTB results.
These recommendations are significant in that they are the first in Korea for NGS and MTB. However, there is also a limitation that the proposed recommendations relied on expert opinions for each core question because concrete scientific evidence is lacking. Nevertheless, we hope that this report provides guidance on applying precision oncology in routine patient care in Korea and accelerating cancer research in clinical research centers.
NotesAuthor Contributions Conceived and designed the analysis: Yoon S, Kim M, Hong YS, Kim HS, Kim ST, Kim J, Yun H, Jang SJ, Zang DY, Kim TW, Kang JH, Kim JH. Collected the data: Yoon S. Contributed data or analysis tools: Yoon S, Kim M, Hong YS, Kim HS, Kim ST, Kim J, Yun H, Yoo C, Ahn HK, Kim HS, Lee IH, Kim IH, Park I, Jeong JH, Cheon J, Kim JW, Yun J, Lim SM, Cha Y, Kim JH. Performed the analysis: Yoon S. Wrote the paper: Yoon S, Kim M, Hong YS, Kim HS, Kim ST, Kim J, Yun H, Kim JH. AcknowledgmentsThis study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1720150).
Fig. 1Korean Precision Medicine Networking Group study of molecular profiling guided therapy based on genomic alterations in advanced solid tumors (KOSMOS) virtual molecular tumor board report. 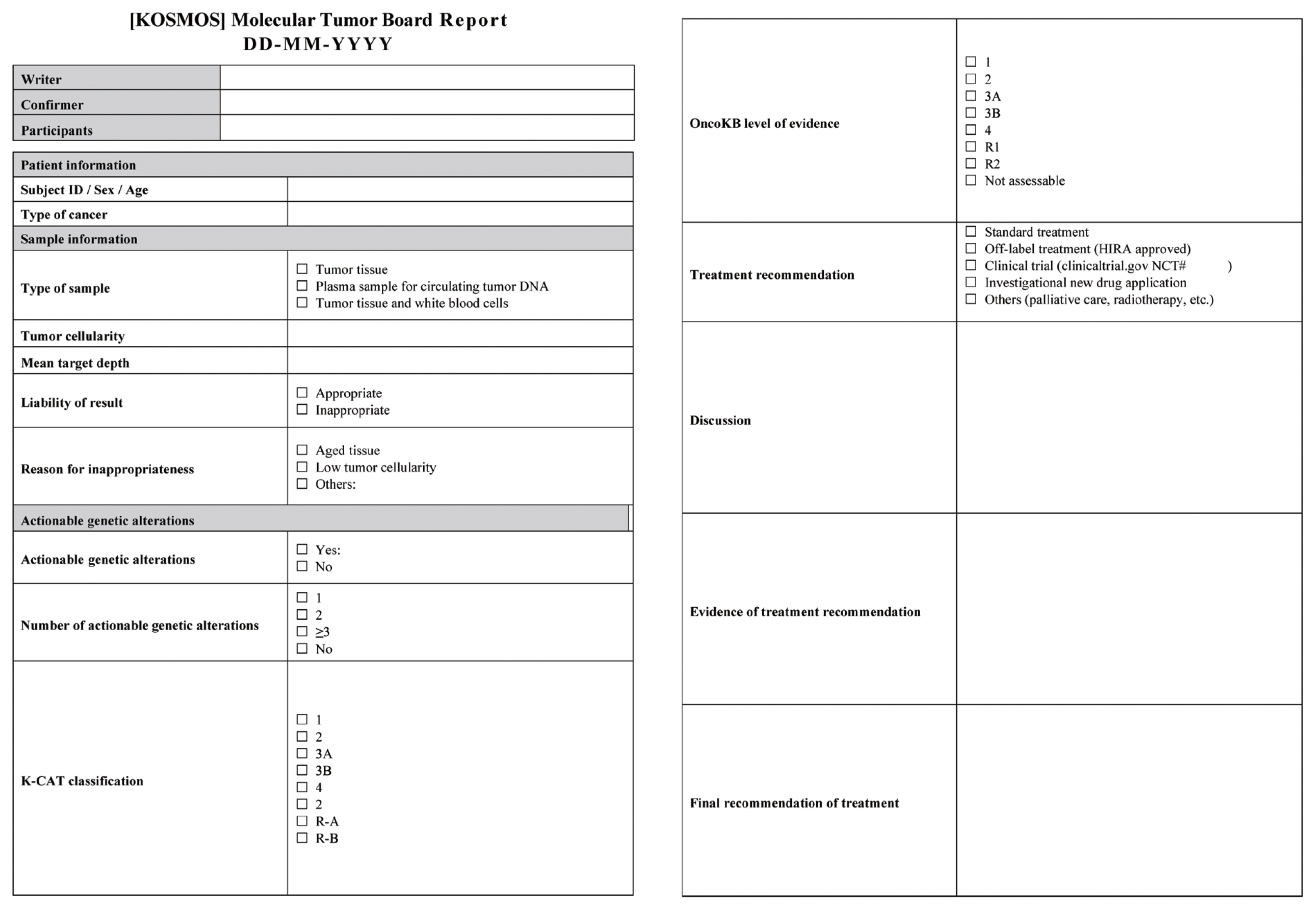
Table 1Key questions
Table 2KPMNG scale of clinical actionability of molecular target (K-CAT) Table 3OncoKB, ESCAT, K-CAT comparison
EMA, European Medicines Agency; ESCAT, European Society for Medical Oncology Scale for Clinical Actionability of molecular Targets; FDA, U.S. Food and Drug Administration; K-CAT, Korean Precision Medicine Networking Group scale of Clinical Actionability of molecular Targets; MFDS, Ministry of Food and Drug Safety; N/A, not accessed. References2. Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526:361–70.
3. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13.
4. Marino P, Touzani R, Perrier L, Rouleau E, Kossi DS, Zhaomin Z, et al. Cost of cancer diagnosis using next-generation sequencing targeted gene panels in routine practice: a nationwide French study. Eur J Hum Genet. 2018;26:314–23.
5. Tobin NP, Foukakis T, De Petris L, Bergh J. The importance of molecular markers for diagnosis and selection of targeted treatments in patients with cancer. J Intern Med. 2015;278:545–70.
6. Lee SH, Lee B, Shim JH, Lee KW, Yun JW, Kim SY, et al. Landscape of actionable genetic alterations profiled from 1,071 tumor samples in Korean cancer patients. Cancer Res Treat. 2019;51:211–22.
7. Grellety T, Lucchesi C, Hostein I, Auzanneau C, Khalifa E, Soubeyran I, et al. High-depth sequencing of paired primary and metastatic tumours: implications for personalised medicine. Eur J Cancer. 2017;84:250–6.
8. Manca A, Paliogiannis P, Colombino M, Casula M, Lissia A, Botti G, et al. Mutational concordance between primary and metastatic melanoma: a next-generation sequencing approach. J Transl Med. 2019;17:289.
9. Crumley SM, Pepper KL, Phan AT, Olsen RJ, Schwartz MR, Portier BP. Next-generation sequencing of matched primary and metastatic rectal adenocarcinomas demonstrates minimal mutation gain and concordance to colonic adenocarcinomas. Arch Pathol Lab Med. 2016;140:529–35.
10. McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15–26.
11. Cho M, Ahn S, Hong M, Bang H, Van Vrancken M, Kim S, et al. Tissue recommendations for precision cancer therapy using next generation sequencing: a comprehensive single cancer center’s experiences. Oncotarget. 2017;8:42478–86.
12. Carithers LJ, Agarwal R, Guan P, Odeh H, Sachs MC, Engel KB, et al. The biospecimen preanalytical variables program: a multiassay comparison of effects of delay to fixation and fixation duration on nucleic acid quality. Arch Pathol Lab Med. 2019;143:1106–18.
13. Chen G, Mosier S, Gocke CD, Lin MT, Eshleman JR. Cytosine deamination is a major cause of baseline noise in next-generation sequencing. Mol Diagn Ther. 2014;18:587–93.
14. Kim S, Park C, Ji Y, Kim DG, Bae H, van Vrancken M, et al. Deamination effects in formalin-fixed, paraffin-embedded tissue samples in the era of precision medicine. J Mol Diagn. 2017;19:137–46.
15. Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic: implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297–312.
16. Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895–902.
17. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017:PO.1700011
18. Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341–65.
19. Heydt C, Wolwer CB, Velazquez Camacho O, Wagener-Ryczek S, Pappesch R, Siemanowski J, et al. Detection of gene fusions using targeted next-generation sequencing: a comparative evaluation. BMC Med Genomics. 2021;14:62.
20. Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27:1236–41.
21. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65.
22. Fancello L, Gandini S, Pelicci PG, Mazzarella L. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J Immunother Cancer. 2019;7:183.
23. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
24. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
25. Luchini C, Bibeau F, Ligtenberg MJ, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232–43.
26. Dedeurwaerdere F, Claes KB, Van Dorpe J, Rottiers I, Van der Meulen J, Breyne J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep. 2021;11:12880.
27. Luchini C, Lawlor RT, Milella M, Scarpa A. Molecular tumor boards in clinical practice. Trends Cancer. 2020;6:738–44.
28. van der Velden DL, van Herpen CM, van Laarhoven HW, Smit EF, Groen HJ, Willems SM, et al. Molecular tumor boards: current practice and future needs. Ann Oncol. 2017;28:3070–5.
29. Larson KL, Huang B, Weiss HL, Hull P, Westgate PM, Miller RW, et al. Clinical outcomes of molecular tumor boards: a systematic review. JCO Precis Oncol. 2021;5:PO.20.00495
30. Pishvaian MJ, Blais EM, Bender RJ, Rao S, Boca SM, Chung V, et al. A virtual molecular tumor board to improve efficiency and scalability of delivering precision oncology to physicians and their patients. JAMIA Open. 2019;2:505–15.
31. Rao S, Pitel B, Wagner AH, Boca SM, McCoy M, King I, et al. Collaborative, multidisciplinary evaluation of cancer variants through virtual molecular tumor boards informs local clinical practices. JCO Clin Cancer Inform. 2020;4:602–13.
32. Health Insurance Review and Assessment ServiceProcess for off-label drug use for chemotherapy [Internet]. Wonju: Health Insurance Review and Assessment Service; 2018. [cited 2021 Dec 8]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030023080000&brdScnBltNo=4&brdBltNo=45637
33. Clinical Trial Policy Division, Pharmaceutical Safety Bureau, Ministry of Food and Drug SafetyGuideline for expanded access program [Internet]. Cheongju: Ministry of Food and Drug Safety; 2020. [cited 2021 Dec 8]. Available from: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14576&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=22
34. Walsh S, de Jong EE, van Timmeren JE, Ibrahim A, Compter I, Peerlings J, et al. Decision support systems in oncology. JCO Clin Cancer Inform. 2019;3:1–9.
35. Blasi L, Bordonaro R, Serretta V, Piazza D, Firenze A, Gebbia V. Virtual clinical and precision medicine tumor boards-cloud-based platform-mediated implementation of multidisciplinary reviews among oncology centers in the COVID-19 era: protocol for an observational study. JMIR Res Protoc. 2021;10:e26220.
36. Kato S, Kim KH, Lim HJ, Boichard A, Nikanjam M, Weihe E, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun. 2020;11:4965.
37. Choi YJ, Choi JY, Kim JW, Lim AR, Lee Y, Chang WJ, et al. Comparison of the data of a next-generation sequencing panel from K-MASTER project with that of orthogonal methods for detecting targetable genetic alterations. Cancer Res Treat. 2021. May. 20[Epub]. https://doi.org/10.4143/crt.2021.218
38. Nakamura Y, Fujisawa T, Taniguchi H, Bando H, Okamoto W, Tsuchihara K, et al. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 2021;112:4425–32.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||