AbstractPurposeWe aimed to investigate the risk factors and patterns of locoregional recurrence (LRR) after radical nephrectomy (RN) in patients with locally advanced renal cell carcinoma (RCC).
Materials and MethodsWe retrospectively analyzed 245 patients who underwent RN for non-metastatic pT3–4 RCC from January 2006 to January 2016. We analyzed the risk factors associated with poor locoregional control using Cox regression. Anatomical mapping was performed on reference computed tomography scans showing intact kidneys.
ResultsThe median follow-up duration was 56 months (range, 1 to 128 months). Tumor extension to renal vessels or the inferior vena cava (IVC) and Fuhrman’s nuclear grade IV were identified as independent risk factors of LRR. The 5-year actuarial LRR rates in groups with no risk factor, one risk factor, and two risk factors were 2.3%, 19.8%, and 30.8%, respectively (p < 0.001). The locations of LRR were distributed as follows: aortocaval area (n=2), paraaortic area (n=4), retrocaval area (n=5), and tumor bed (n=11). No LRR was observed above the celiac axis (CA) or under the inferior mesenteric artery (IMA).
ConclusionTumor extension to renal vessels or the IVC and Fuhrman’s nuclear grade IV were the independent risk factors associated with LRR after RN for pT3–4 RCC. The locations of LRR after RN for RCC were distributed in the tumor bed and regional lymphatic area from the bifurcation of the CA to that of the IMA.
IntroductionKidney cancer is a rare disease accounting for approximately 3.8% of all new malignancies [1], and renal cell carcinomas (RCCs) comprise approximately 85% of kidney tumors [2]. One of the standard treatments for non-metastatic RCCs, especially those in the advanced stage, is radical nephrectomy (RN) [3]. However, 5%–15% of patients who undergo RN experience disease recurrences and among high-risk patients, even higher recurrence rates (up to 35%–40%) have been reported [4,5]. Therefore, there is an increasing need for effective adjuvant therapies among these patients. However, despite ongoing clinical trials, no standardized adjuvant therapy currently exists [3].
Although distant metastasis is the main pattern of RCC recurrence after RN, locoregional recurrence (LRR) is a significant recurrence pattern in patients with risk factors, including those diagnosed with locally advanced disease [6]. In addition, patients who develop LRR after RN show a dismal prognosis because salvage treatments are not feasible in many cases [6]. To reduce the incidence of LRR, extended locoregional therapies secondary to RN such as regional lymph node dissection (LND) or local adjuvant therapy are considered for high-risk patients. However, there are no established strategies to reduce the incidences LRRs other than RN [3,7]. For LND, no consensus currently exists on its indication, value, or extent in patients with RCC [8,9]. In contrast, the role of adjuvant radiation therapy (RT) in RCC has been investigated in previous trials and proven to be negative [10,11]. However, these negative results may have resulted from the heterogeneity in previous study populations, which included patients with early stage RCC who were at low risk for LRR after RN [10–12]. Therefore, careful investigation of risk factors for LRR after RN is needed to suggest the potential indication of the extended treatment. Furthermore, the adequate extent of locoregional therapy must be established according to the patterns of LRR after RN so that high-risk LRR could be specifically managed and redundant toxicity from unnecessary treatment could be avoided. In this study, we investigated the prognostic value, risk factors, and patterns of LRR after RN in patients with locally advanced RCC to recommend the potential indications and adequate extent of locoregional therapy in high-risk patients.
Materials and Methods1. PatientsThe inclusion criteria of this study were as follows: (1) RN for RCC, (2) non-metastatic RCC, (3) pathologic T3 or T4 category, (4) no neoadjuvant treatment, and (5) no other uncontrolled malignancy. We retrospectively reviewed the medical records of 948 patients who underwent RN for RCC from January 2006 to January 2016. Among them, 703 patients were excluded due to the presence of metastasis (n=120), pathologic T1 or T2 category (n=563), neoadjuvant treatment (n=4), follow-up loss (n=3), and other uncontrolled malignancy (n=13). In total, 245 patients were included in the analyses.
2. TreatmentsThe RNs were performed via open surgery (n=116), conventional laparoscopic surgery (n=89), or hand-assisted laparoscopic surgery (n=40). According to the surgeon’s decision, LND or lymph node sampling was performed in 85 patients. Adjuvant systemic agents were administered to 12 patients (pazopanib, n=4; axitinib, n=8).
3. Statistical analysesLocoregional control (LRC), distant metastasis-free survival (DMFS), and overall survival (OS) were defined as the interval from RN to LRR, distant metastasis, and death or the last follow-up (if no relevant event occurred), respectively. LRRs were defined as the recurrences in area of kidney beds and the regional lymph nodes including renal hilar, precaval, paracaval, retrocaval, interaortocaval, and paraaortic lymph nodes. The boundary of regional lymphatic area was considered from the diaphragmatic aortic hiatus to the upper border of the origin of the inferior mesenteric artery (IMA) longitudinally and around the renal vein, inferior vena cava (IVC) and abdominal aorta with 10 mm margin laterally and antero-posteriorly [12–14]. The survival curves were plotted using the Kaplan-Meier method. The log-rank test was used for univariate analyses. Cox regression was used in multivariate analyses of variables with p ≤ 0.200 in the univariate analysis. Phi and Cramer’s V were calculated for evaluation of collinearities among the variables to exclude inappropriate variables for the multivariate analyses. A two-sided p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 24.0 (IBM Corp., Armonk, NY).
4. Anatomical mapping of LRRAn abdominopelvic computed tomography (CT) scan of a control patient without RCC was used as reference image. First, we marked the celiac axis (CA), superior mesenteric artery, and IMA on the reference CT scan. Then, we mapped all LRRs manually at the equivalent locations on the reference CT scan considering the anatomical relations to the locations of the pre-RN kidney, vessels, and adjacent muscles. To reduce volume effects, each LRR was transformed to a 5-mm point at the epicenter of the recurrent tumor. Registration of CT images and LRR mapping were performed using Pinnacle TPS (Philips Radiation Oncology System, Fitchburg, WI).
Results1. Patients’ characteristicsPatient demographics are summarized in Table 1. The patients’ median age was 58 years (range, 15 to 91 years). The median longest tumor diameter was 7.0 cm (range, 2 to 18 cm). Only one patient (0.4%) had RCC of clear cell type with cystic change. The median follow-up duration was 56 months (range, 1 to 128 months).
2. Treatment outcomesThe 5-year actuarial rates of LRC, DMFS, and OS were 89.8%, 60.4%, and 82.1%, respectively (Fig. 1). The median time of LRR was 14 months (range, 2 to 54 months). The patterns of the first recurrence are shown in the S1 Table, which shows the rates of overall LRR (6.9%) and distant recurrence as the first recurrence event (38.3%). Seven of the 10 regional recurrences occurred in patients who did not undergo LND or lymph node sampling during RN. LRR as the first recurrence event was significantly associated with actuarial rates of subsequent distant metastasis (p=0.003) and survival (p < 0.001) (Fig. 2).
The results of univariate and multivariate analyses for LRC are presented in Table 2. Tumor extension to renal vessels or the IVC (p=0.036) and Fuhrman’s nuclear grade IV (p=0.014) were identified as independent risk factors associated with LRC in the multivariate analyses. The 5-year actuarial LRR rates in groups with no risk factor (low-risk), one risk factor (intermediate-risk), and two risk factors (high-risk) were 2.3%, 19.8%, and 30.8%, respectively (p < 0.001) (Fig. 3). The 5-year DMFS rates were calculated as 71.5%, 45.8%, and 39.5% in the low-, intermediate-, and high-risk groups, respectively (p < 0.001) (Fig. 3). The 5-year OS rates were also significantly different among the risk groups, with values of 91.6%, 72.9%, and 71.5% in the low-, intermediate-, and high-risk groups, respectively (p=0.004) (Fig. 3).
3. Anatomical mapping of LRRThe anatomical map of LRR is shown in Fig. 4. The LRR sites in patients with a right-side RCC were the aortocaval area (n=2), retrocaval area (n=3), and tumor bed (n=9). In the tumor bed, LRR occurred at sites adjacent to muscles, including the back muscle (n=4) and crus (n=4), except in one patient with a primary right RCC invading the IVC, in whom the LRR occurred inside the IVC. In contrast, the LRR sites in patients with left-side RCC were the paraaortic area (n=4), retrocaval area (n=2), and tumor bed (near the adjacent psoas muscle or crus) (n=2). No LRR was observed above the CA or below the IMA.
DiscussionPrevious studies have reported that the incidence of LRR after RN for RCC ranges from 1.8% to 27% [15]. These diverse outcomes have resulted from the heterogeneity in disease status, such as stage and histologic grade [6]. The LRR rates following RN in patients with early stage RCC were reported to be around or less than 5%, whereas higher LRR rates (over 30%) were reported in those with locally advanced RCC [6,15,16]. In the present study, the 5-year actuarial LRR rate was 10.2%. Furthermore, our study showed that LRR as the first recurrence event is significantly associated with subsequent distant metastasis or death. Therefore, efforts to reduce the incidence of LRR after RN for locally advanced RCC should be made in patients with risk factors, despite distant metastasis being the main pattern of recurrence after RN for locally advanced RCC [15,17].
Previous reports have suggested several predictive models for the risk of RCC relapse following surgical resection, with the predictor variables including pathological stage, nuclear grade and presence of necrosis, major dimensions of the tumor, and/or performance status [18–21]. However, these variables are related not only to LRR but also to distant metastasis. Little research has been conducted on prognosticators associated with LRR alone. This is probably because LRR is not the major failure pattern of RCC following RN, as evidenced by the 5-year rate of LRR as the first recurrence event of 10.2% in our study. However, LRR could be a concern in patients with certain risk factors. In the present study, tumor extension to renal vessels or the IVC and Fuhrman’s nuclear grade IV were identified as independent risk factors. As seen in the groups categorized using meaningful risk factors, the LRR rates in the low-, intermediate-, and high-risk groups were 2.3%, 19.8%, and 30.8%, respectively. Jhavar et al. [6] have suggested that larger tumor size, stage III/IV, Fuhrman’s nuclear grade, tumor invasion of the renal vein, perinephric fat invasion, and the combinations of these factors were associated with LRR following RN for RCC. Previous predictive models included pathologic staging with broad subcategories [18–21]; careful risk stratification according to staging subcategories would be necessary for the selection of LRR risk groups, because the impact of LRR in each subcategory could be different.
To reduce LRR rates after RN, LND could be performed during RN. Previous studies on the role of LND during surgery for RCC showed conflicting results [22]. Furthermore, the only prospective study comparing nephrectomy alone and nephrectomy with LND failed to demonstrate the benefit of LND in cancer control [23]. However, the characteristics of patients included in this study were heterogenous, and a significant proportion of the participants had early stage and low grade disease, which has negligible LRR risk [24]. In contrast, LND is potentially beneficial to patients with larger tumors, locally advanced disease, high Fuhrman grade, sarcomatoid features, or tumor necrosis [22]. Adjuvant RT is another tool that could considerably enhance LRC after RN. Reports about the role of adjuvant RT after RN in improving LRC and survival are limited. Early prospective clinical trials have evaluated the role of adjuvant RT after nephrectomy for RCC and showed no benefit of RT for local tumor control and high treatment related mortality [10,11]. However, the inclusion criteria in these studies was a point of criticism because the study population included some low-risk patients with stage II RCC who exhibited low LRR rates after nephrectomy alone. In contrast to these earlier clinical trials, several retrospective studies have recently reported the benefits of adjuvant RT for RCC [25–29]. From the results, Tunio et al. [30] suggested a new trial to evaluate adjuvant RT in patients with RCC using the following eligibility criteria: tumor size > 5 cm, positive margins or postoperative gross residual disease, perinephric fat invasion, capsule infiltration, renal vein or IVC invasion, lymph node metastasis, and/or high grade histology. These criteria include risk factors identified in our study as well as those identified in the study by Jhavar et al. [6]. However, to develop a more robust indication for LND or adjuvant RT, a large-scale study to analyze the risk factors of LRR after RN for RCC is required.
In addition to potential indications, the scope of extended locoregional therapy is another issue. Campi et al. [12] reported an overview of the most commonly dissected templates in LND for RCC based on previously published work. The authors described that for right-sided tumors, LND mostly included the renal hilar, paracaval, and precaval nodes, whereas for left-sided tumors, the renal hilar, preaortic, and paraaortic nodes were included. The most commonly proposed extension was from the crus of the diaphragm to the aortic bifurcation longitudinally. However, considering the LRR map in our study, the suggested area for LND is beyond the scope of LRR, since no LRR above the CA or below the IMA was reported. In addition to evaluating LND, Tunio et al. [30] proposed the target volume for adjuvant RT as follows: the renal bed from the upper pole to lower pole level of the opposite kidney and lymph nodes only if lymph node metastasis is histologically confirmed. However, considering our LRR map, the sites of LRR were not distributed in the entire renal bed but only in the tumor bed near adjacent muscles. The delineation of area at the tumor before RN, concerning the adjacent muscle, seems to be sufficient for the target volume of tumor bed. Furthermore, 70% of regional recurrences as the first recurrence occurred in the patients who had not undergone histological confirmation of lymph node metastases. Therefore there are discrepancies between the previously suggested scope for extended locoregional therapy (such as additional LND or adjuvant RT) and the LRR map in the present study. Recommendations on adequate scope may need to be revised based on the locational distribution of LRRs after RN for RCC.
There are several limitations to the present study. First, this was a retrospective study, and thus, some selection biases cannot be completely eliminated. Second, the sample sizes for some of the variables were so small that the observed statistical significance for the association with LRC may not always hold true. In conclusion, tumor extension to renal vessels or the IVC and/or Fuhrman’s nuclear grade IV are the independent risk factors associated with LRR after RN for pathologic T3–4 RCC. The locations of LRR after RN for RCC were distributed in the tumor bed and regional lymphatic area from the bifurcation of CA to that of the IMA. Further research may be needed to confirm the role of LND or adjuvant RT in reducing the LRR rate and improving survival after RN for locally advanced RCC.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by our institutional review board (SMC 2019-08-148), and the need for informed consent was waived. Author Contributions Conceived and designed the analysis: Park W, Seo SI. Collected the data: Yoo GS, Jeong BC, Jeon HG, Kang M, Seo SI, Jeon SS, Lee HM, Choi HY, Park BK, Kim CK, Park SY, Kwon GY. Contributed data or analysis tools: Yoo GS. Performed the analysis: Yoo GS. Wrote the paper: Yoo GS, Park W, Seo SI. Supervision: Park W, Seo SI. Critical review: Pyo H, Jeong BC, Jeon HG, Kang M, Jeon SS, Lee HM, Choi HY, Park BK, Kim CK, Park SY, Kwon GY. Fig. 1Survival curves for locoregional control (A), distant metastasis-free survival (B), and overall survival (C). 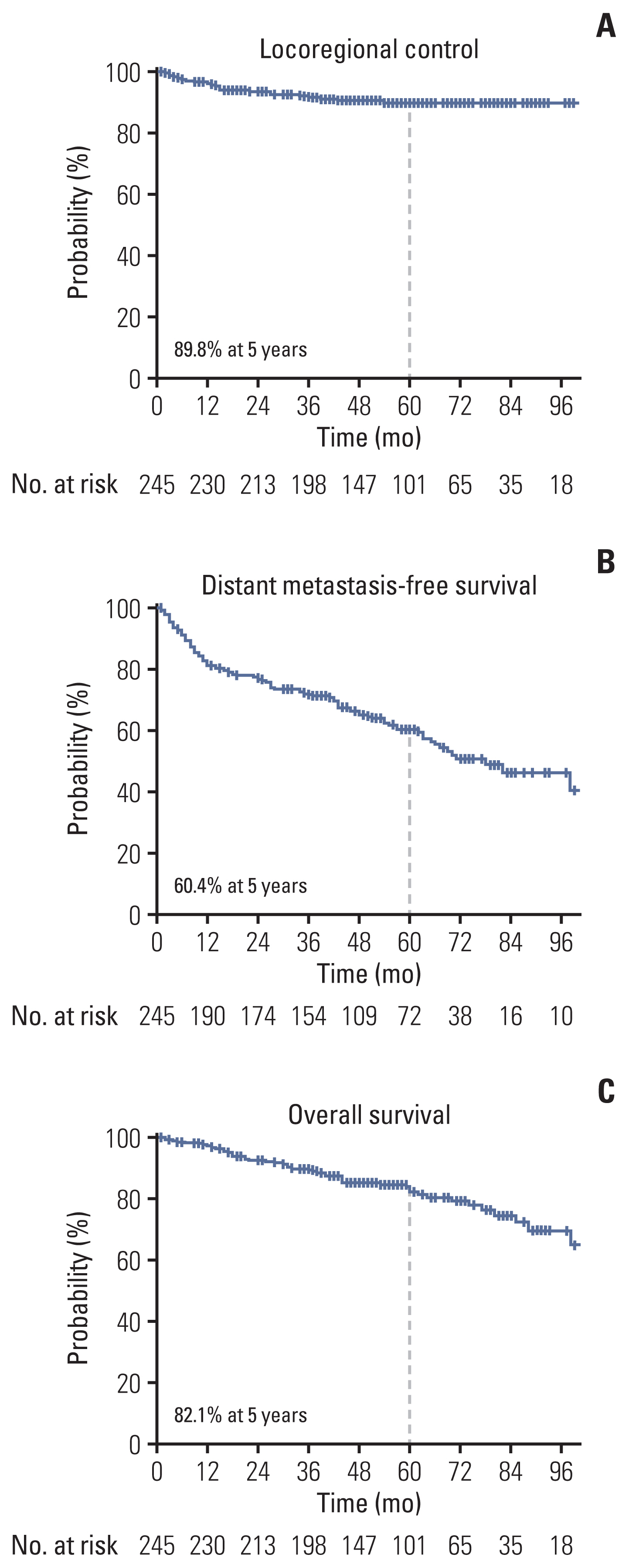
Fig. 2Survival curves for distant metastasis-free survival (A) and overall survival (B) according to the events of locoregional recurrence (LRR) as the first recurrence. 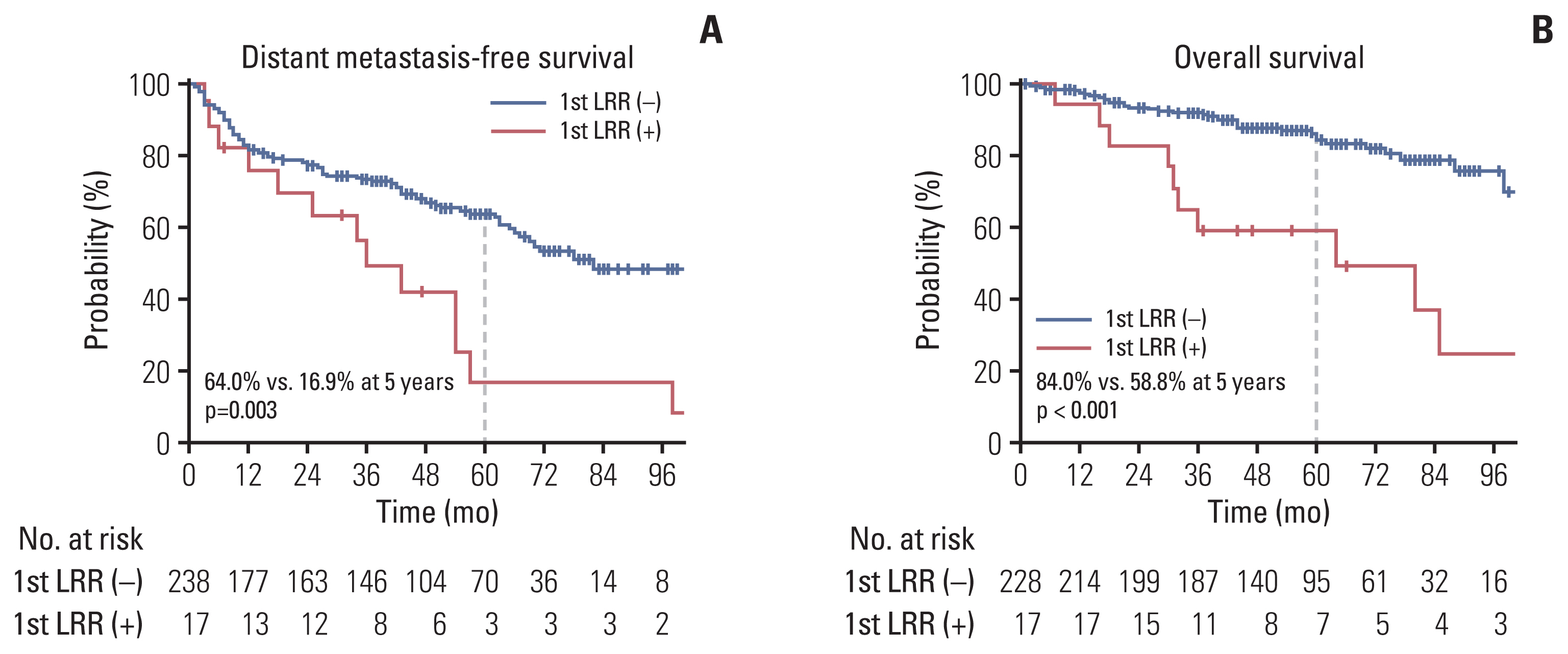
Fig. 3Survival curves for locoregional control according to the risk group. Locoregional recurrence (A), distant metastasis-free survival (B), and overall survival (C). 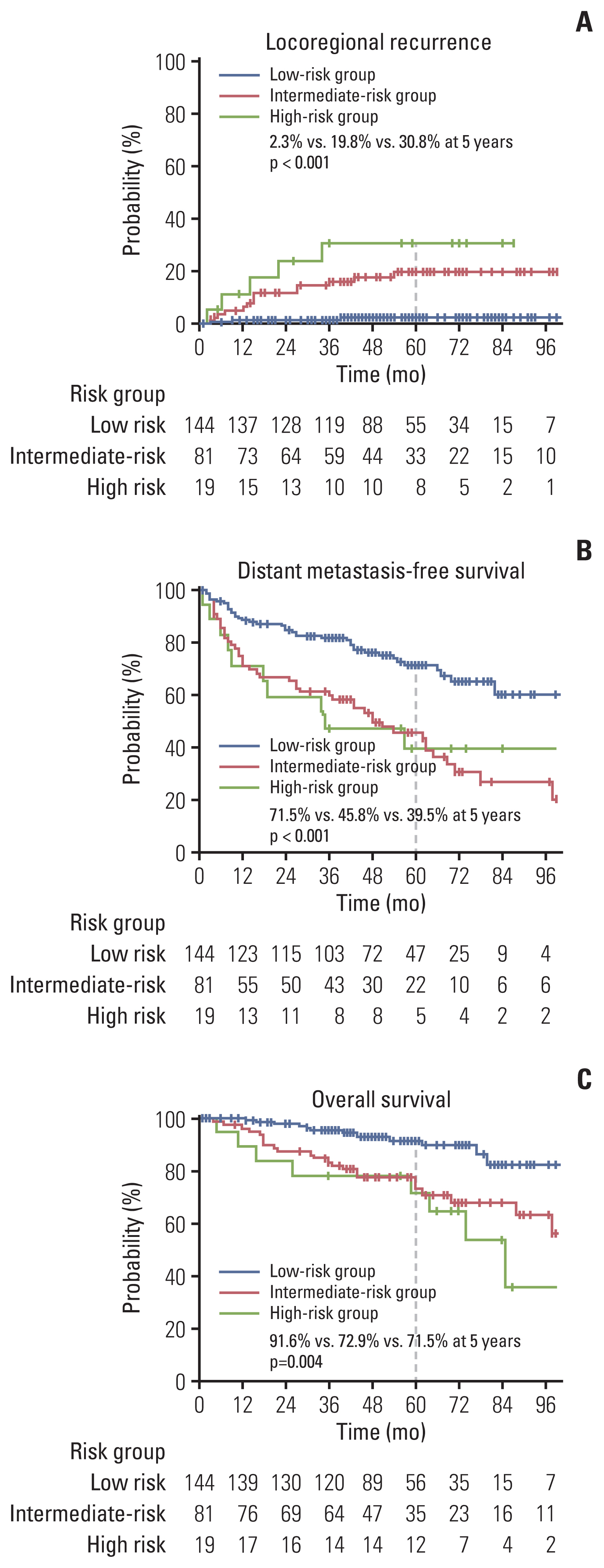
Fig. 4Anatomical map of locoregional recurrence (LRR). Blue and yellow dots represent the LRR locations for right- and left-renal cell carcinomas, respectively. Red circled dots represent the celiac axis (top), superior mesenteric artery (middle), and inferior mesenteric artery (bottom), respectively. 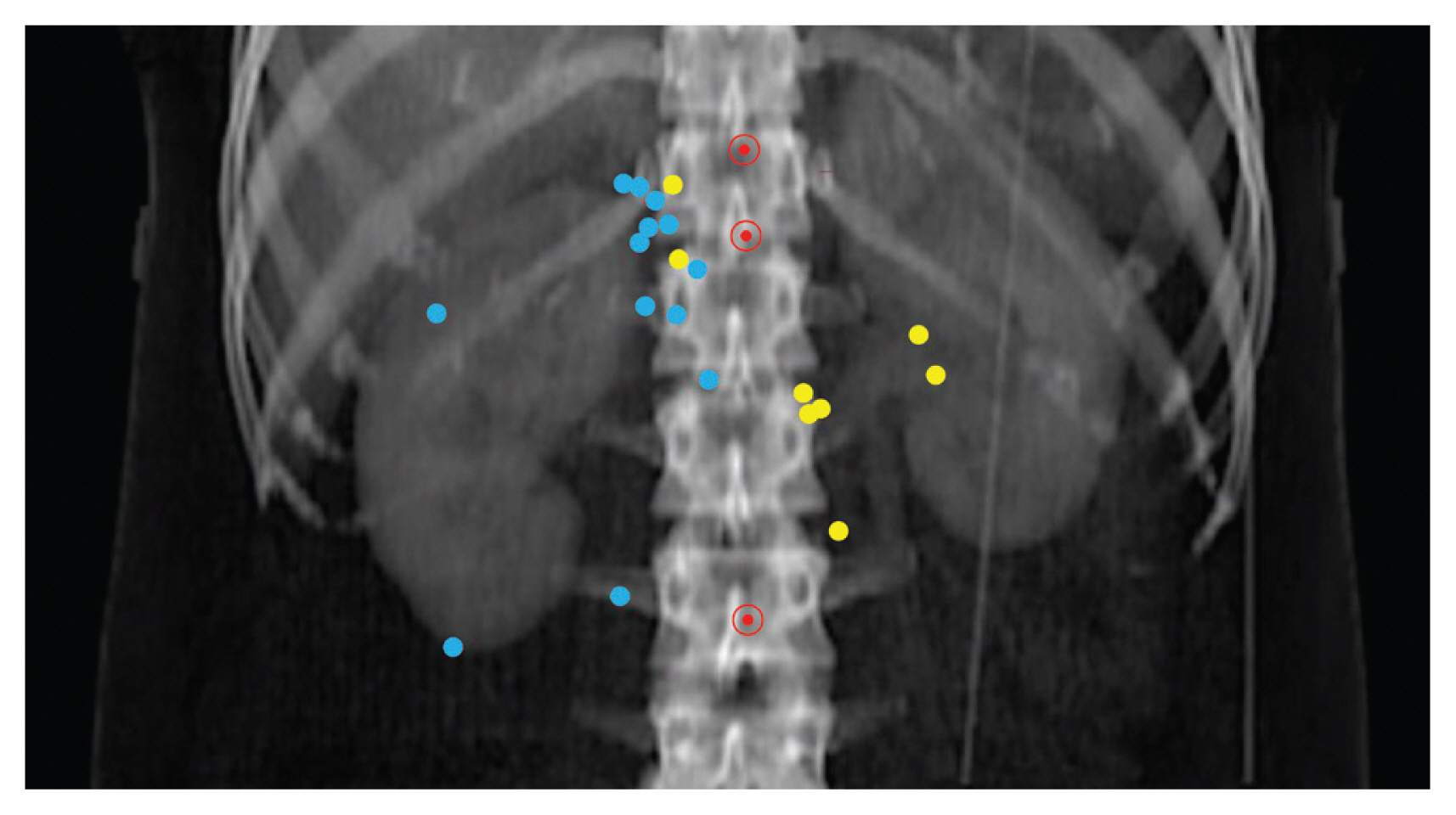
Table 1Demographics of the patients (n=245) Table 2Univariate and multivariate analyses for LRC
References2. Lipworth L, Morgans AK, Edwards TL, Barocas DA, Chang SS, Herrell SD, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. 2016;117:260–5.
3. National Comprehensive Cancer NetworkKidney cancer (version 1.2020) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [cited 2020 Jun 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
4. Kim SP, Weight CJ, Leibovich BC, Thompson RH, Costello BA, Cheville JC, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011;78:1101–6.
5. Lenis AT, Donin NM, Johnson DC, Faiena I, Salmasi A, Drakaki A, et al. Adjuvant therapy for high risk localized kidney cancer: emerging evidence and future clinical trials. J Urol. 2018;199:43–52.
6. Jhavar S, Swanson G, Pruszynski J. Risk factors for locoregional relapse after radical nephrectomy. Asia Pac J Clin Oncol. 2018;14:192–7.
7. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30:706–20.
8. Ljungberg B, Albiges L, Bedke K, Bex A, Capitanio U, Giles RH, et al. European Association of Urology guidelines on renal cell carcinoma [Internet]. Arhem: European Association of Urology; 2019. [cited 2020 Jun 7]. Available from: https://uroweb.org/guideline/renal-cell-carcinoma/
9. Ward RD, Tanaka H, Campbell SC, Remer EM. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics. 2018;38:2021–33.
10. Finney R. The value of radiotherapy in the treatment of hypernephroma: a clinical trial. Br J Urol. 1973;45:258–69.
11. Kjaer M, Iversen P, Hvidt V, Bruun E, Skaarup P, Bech Hansen J, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma: a study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285–9.
12. Campi R, Sessa F, Di Maida F, Greco I, Mari A, Takacova T, et al. Templates of lymph node dissection for renal cell carcinoma: a systematic review of the literature. Front Surg. 2018;5:76.
13. Amin MB, Edge SB, Green FL. AJCC cancer staging manual. 8th ed.. New York: Springer; 2017.
14. Haijun Y, Qiuji W, Zhenming F, Yong H, Zhengkai L, Conghua X, et al. A new approach to delineating lymph node target volumes for post-operative radiotherapy in gastric cancer: a phase II trial. Radiother Oncol. 2015;116:245–51.
15. Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8:1–7.
16. Liu G, Ma Y, Wang S, Han X, Gao D. Laparoscopic versus open radical nephrectomy for renal cell carcinoma: a systematic review and meta-analysis. Transl Oncol. 2017;10:501–10.
17. Fayek IS, Habashy HF, Habashy NF. Isolated locoregional recurrence after radical nephrectomy for renal cell carcinoma: a study of 22 patients. J Egypt Natl Canc Inst. 2014;26:161–6.
18. Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–57.
19. Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71.
20. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400.
21. Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–22.
22. Capitanio U, Leibovich BC. The rationale and the role of lymph node dissection in renal cell carcinoma. World J Urol. 2017;35:497–506.
23. Blom JH, van Poppel H, Marechal JM, Jacqmin D, Schroder FH, de Prijck L, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28–34.
24. Kates M, Lavery HJ, Brajtbord J, Samadi D, Palese MA. Decreasing rates of lymph node dissection during radical nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2012;19:2693–9.
25. Ulutin HC, Aksu G, Fayda M, Kuzhan O, Tahmaz L, Beyzadeoglu M. The value of postoperative radiotherapy in renal cell carcinoma: a single-institution experience. Tumori. 2006;92:202–6.
26. Safwat A, Shouman T, Hamada E, El-Naggar H, Mohamed NH. Post operative radiotherapy improves disease free but not overall survival in high risk renal cell carcinoma patients. J Egypt Natl Cancer Inst. 2000;12:17–22.
27. Makarewicz R, Zarzycka M, Kulinska G, Windorbska W. The value of postoperative radiotherapy in advanced renal cell cancer. Neoplasma. 1998;45:380–3.
28. Kao GD, Malkowicz SB, Whittington R, D’Amico AV, Wein AJ. Locally advanced renal cell carcinoma: low complication rate and efficacy of postnephrectomy radiation therapy planned with CT. Radiology. 1994;193:725–30.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||